Amino methanol derivant and salt compound thereof as well as synthesizing method and medical application thereof
A technology of salt compounds and aminomethanol, which is applied in the preparation of aminohydroxyl compounds, active ingredients of heterocyclic compounds, organic chemical methods, etc., can solve the problems of non-research and development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
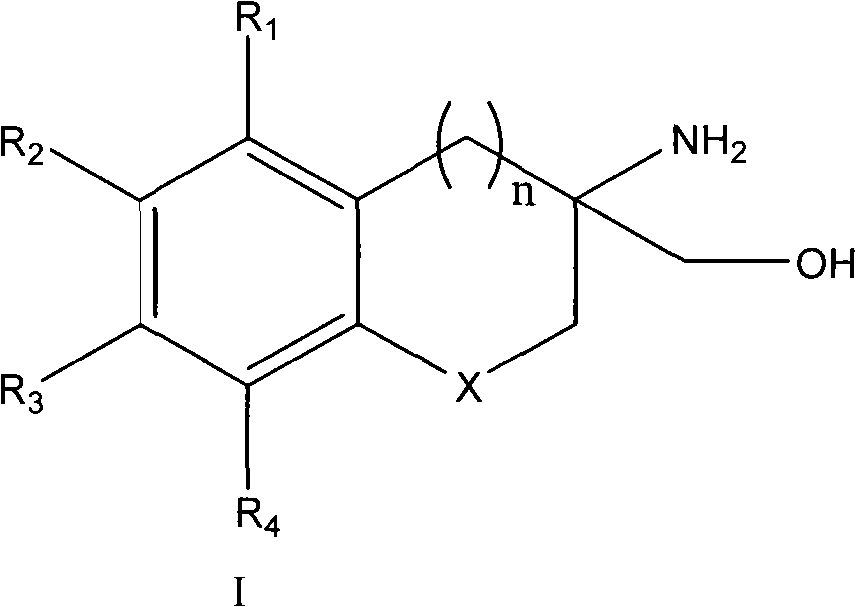
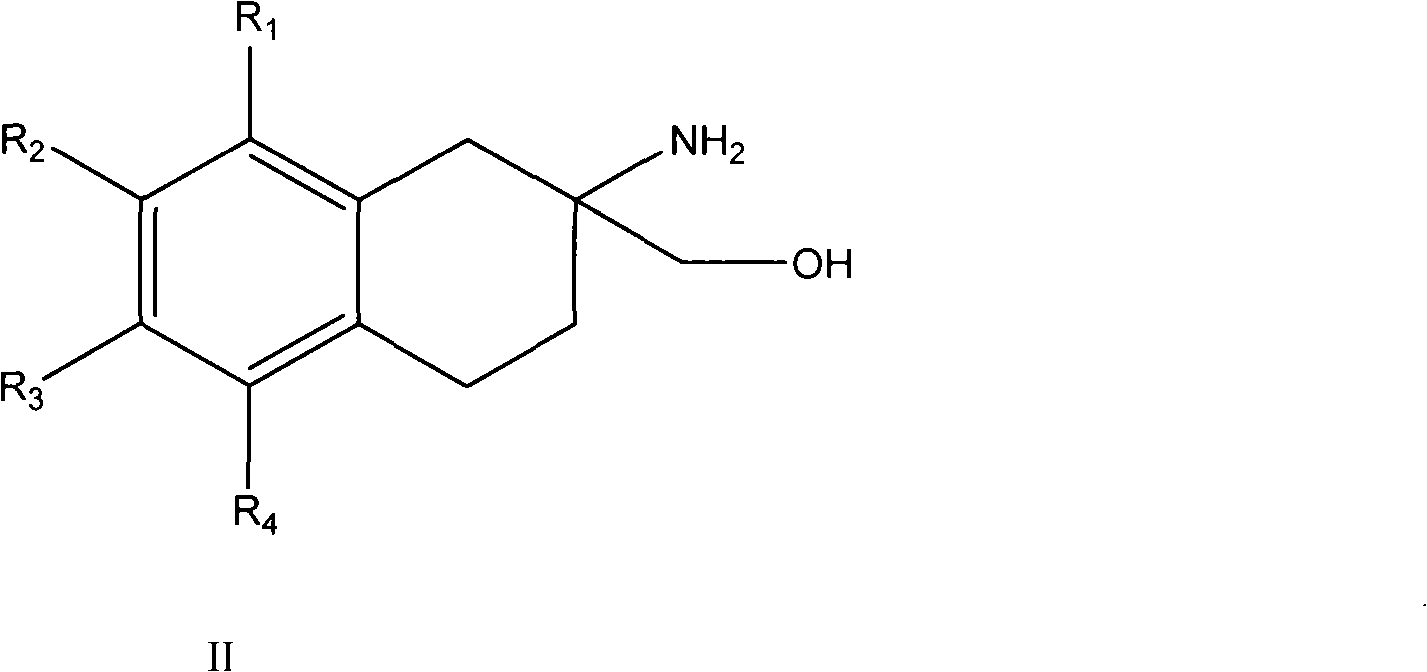
[0106] One embodiment of the present invention, such as the compound of formula II:
[0107]
[0108] in:
[0109] R1 = methyl, chlorine, bromine, fluorine;
[0110] R2 = H, C 4 -C 6 -C 8 Alkyl, Heptyloxy, Phenoxy, 4-Methoxyphenoxy, 4-(Benzyloxy)phenoxy, Phenyl, 6-Methoxyhexyl, 4-(Benzyloxy) Phenylthio, 3-methoxyphenoxy, 3-(benzyloxy)phenoxy, 3-(benzyloxy)phenylthio, halogen, 6-methoxyhexyl, phenoxy, 2-chlorophenoxy, 3-chlorophenoxy, 4-chlorophenoxy, 3-methoxyphenoxy, 3-(benzyloxy)phenoxy, phenyl, 3-(benzyl Oxy)phenylthio, 6-methoxyhexyl;
[0111] R3=H, C 4 -C 6 -C 8 Alkyl, Heptyloxy, Phenoxy, 4-Methoxyphenoxy, 4-(Benzyloxy)phenoxy, Phenyl, 6-Methoxyhexyl, 4-(Benzyloxy) Phenylthio, 3-methoxyphenoxy, 3-(benzyloxy)phenoxy, 3-(benzyloxy)phenylthio, halogen, 6-methoxyhexyl, phenoxy, 2-chlorophenoxy, 3-chlorophenoxy, 4-chlorophenoxy, 3-methoxyphenoxy, 3-(benzyloxy)phenoxy, phenyl, 3-(benzyl Oxy)phenylthio, 6-methoxyhexyl;
Embodiment 1
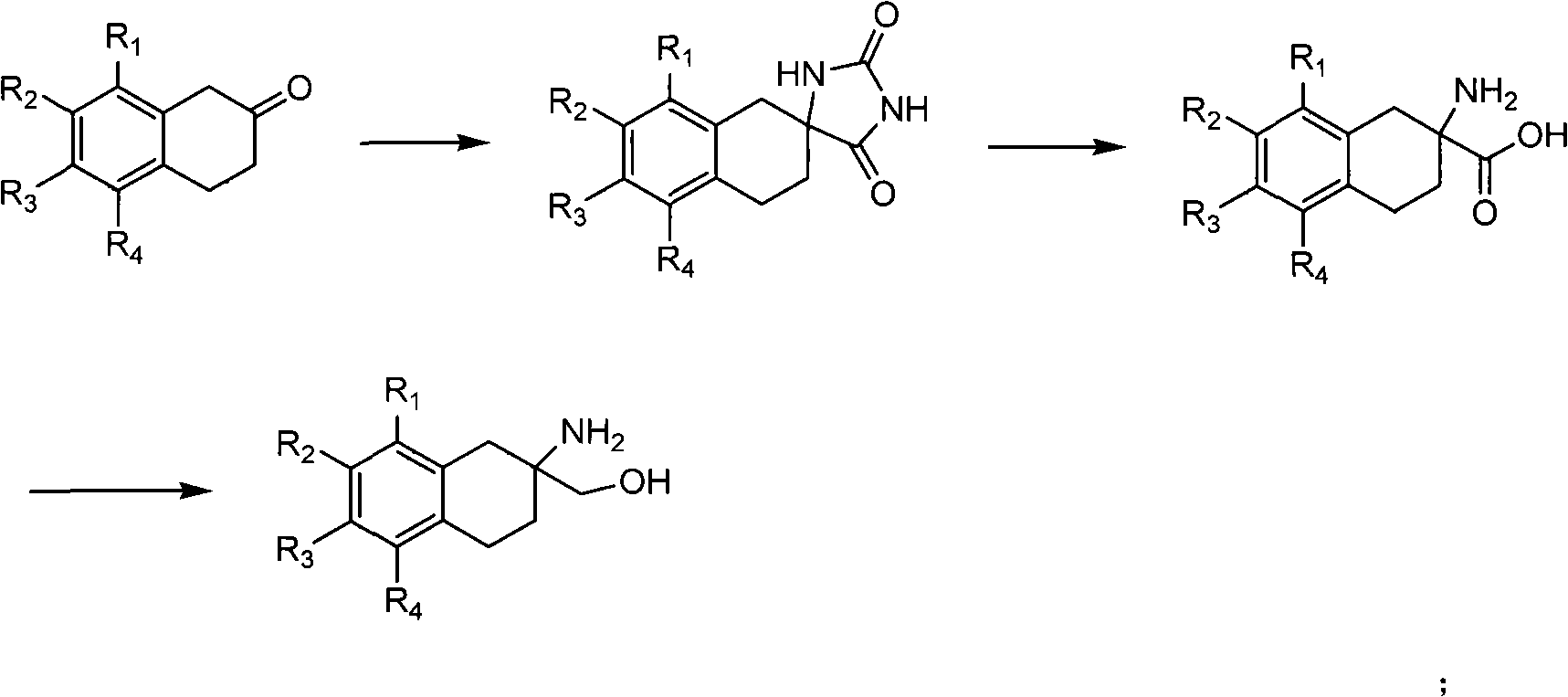
[0124] The synthetic method of 2-amino-1,2,3,4-tetrahydro-2-naphthalenemethanol (FC-001) and its hydrochloride (FC-002) (hereinafter referred to as FC-001, FC-002) is as follows Show:
[0125]
[0126] Synthesis of 3',4'-dihydro-spiro[imidazoline-4,2'(1'H)-naphthalene]-2,5-dione
[0127] Take 8g (54.8mmol) of 2-tetralone, 4g (61.5mmol) of potassium cyanide, 48g (500mmol) of ammonium carbonate, and 320mL of 50% ethanol solution, heat and reflux for 2h, evaporate the ethanol, filter, and wash the filter cake with water until Neutral, dried to give 8.6 g of white solid 3',4'-dihydro-spiro[imidazoline-4,2'(1'H)-naphthalene]-2,5-dione, yield 73%; mp 150 -152°C; 1 H NMR (400MHz, DMSO) δ: 10.69(s, 1H, -NH-), 8.29(s, 1H, -NH-), 7.08-7.13(m, 4H, 4×-ArH), 3.12(d, 1H , J=17.2Hz, -CH-), 2.88-2.97 (m, 2H, -CH 2-), 2.76(d, 1H, J=16.8Hz), 1.94-1.98(m, 1H, -CH-), 1.82-1.84(m, 1H, -CH-); 13 C NMR (100 MHz, DMSO) δ: 178.1, 156.3, 134.8, 132.6, 128.9, 128.5, 125.9, 125.8, 60.7, 36.8, 30....
Embodiment 2
[0136] 2-amino-7-n-butyl-1,2,3,4-tetrahydro-2-naphthalenemethanol (FC-003) and its hydrochloride (FC-004) (hereinafter referred to as FC-003, FC-004 ) is synthesized as follows:
[0137]
[0138] Synthesis of 7-bromo-2-tetralone
[0139] Take 20g (94mmol) of m-bromophenylacetic acid, 240mL of 1,2-dichloroethane, 21mL (296mmol) of thionyl chloride, heat to reflux for 4h, spin the solvent, add 100mL of dichloromethane, and add the above acid chloride solution to Into a mixed solution of 28g (209mmol) of anhydrous aluminum chloride and 300mL of dichloromethane, feed ethylene at a temperature lower than 0°C for reaction, after the reaction (about 4h), add 200mL of water, extract with dichloromethane, The organic phase was washed successively with 1N HCl and saturated sodium carbonate solution, dried over anhydrous magnesium sulfate, and evaporated to remove the solvent. Silica gel column chromatography (developing solvent: ethyl acetate:petroleum ether=1:5) gave white solid 7-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com