Preparation method of 3,5-cynarin methyl ester and medicament composition thereof
A technology of caffeoylquinic acid methyl ester and methyl esterification, which is applied to the preparation of carboxylic acid esters, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problem of uncertain covalent closed-loop HBV DNA clearance, side effects, and easy Rebound and other problems, to achieve significant anti-influenza virus activity, strong inhibitory effect, and good anti-influenza virus effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
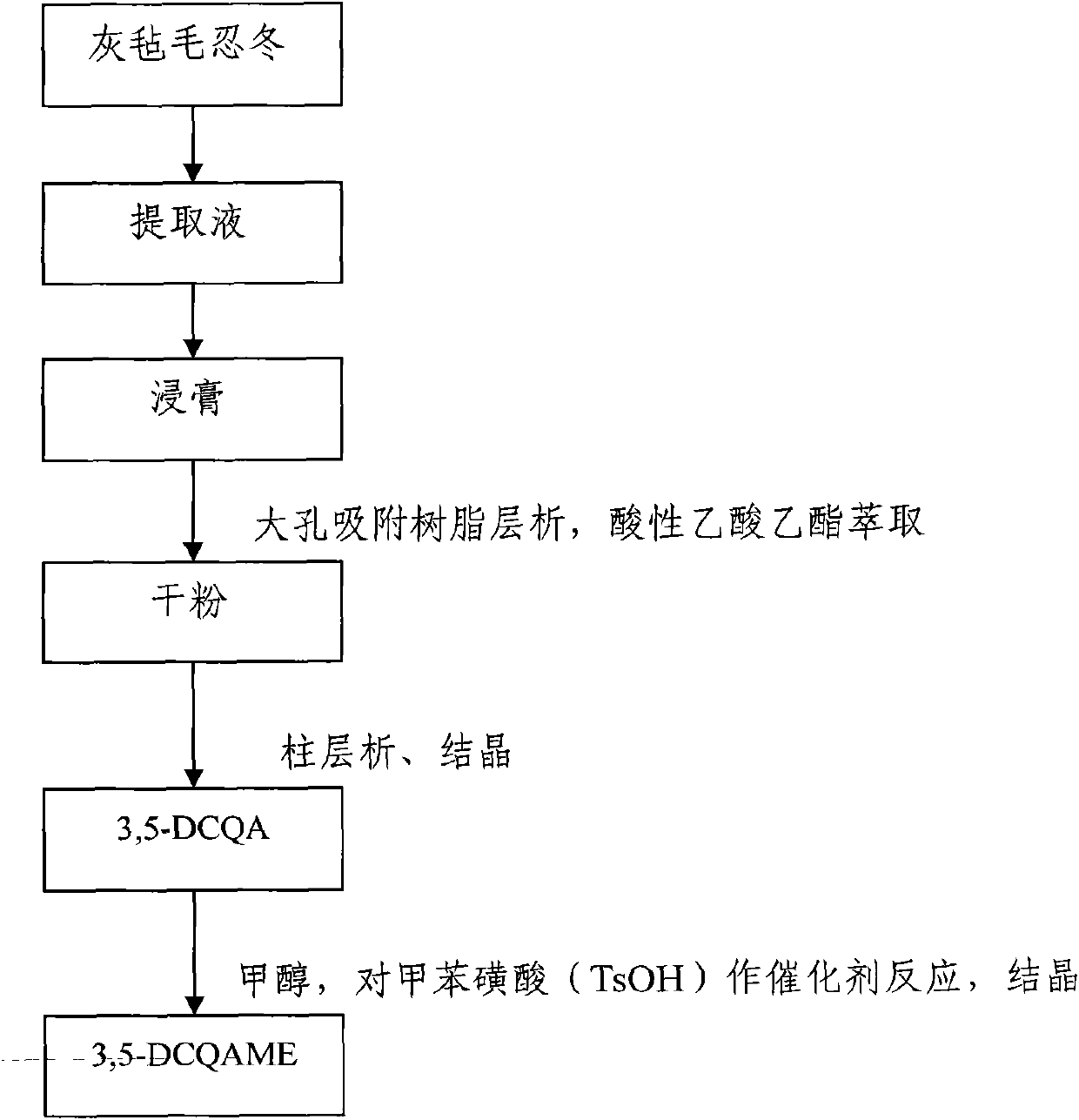
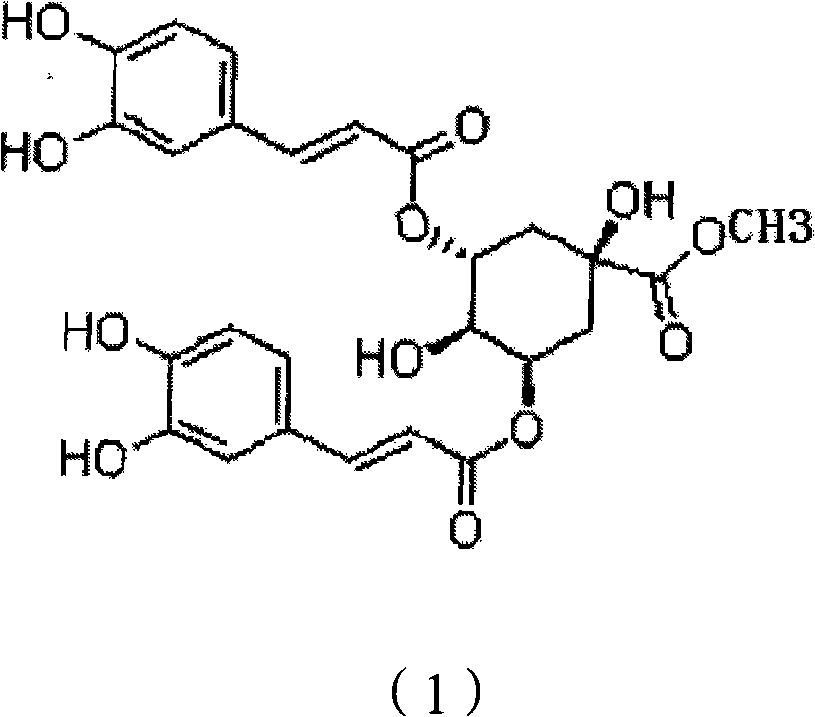
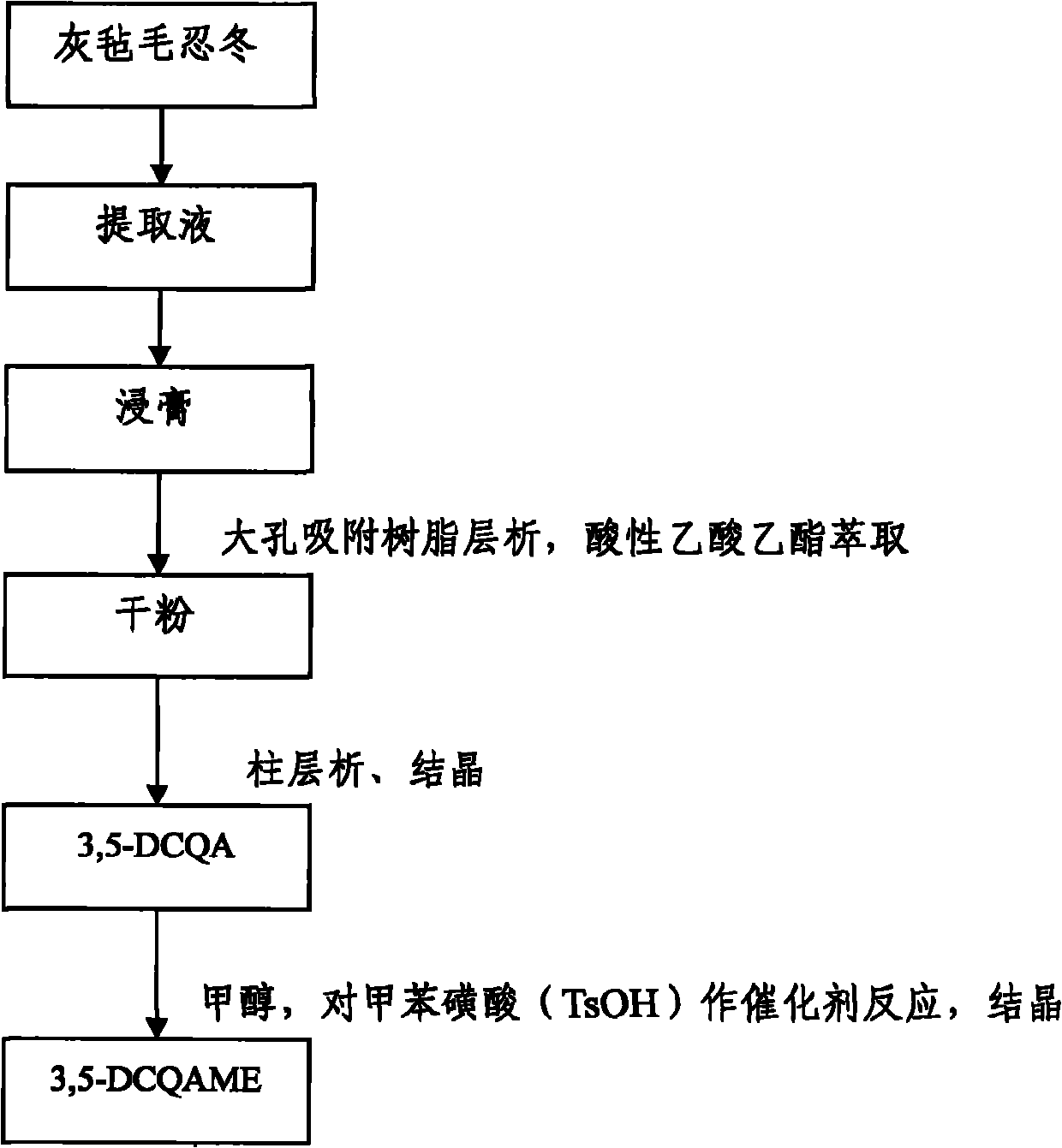
[0045] Embodiment 1: 3, the preparation of 5-DCQAME
[0046] Take 20kg of dried flower buds of Lonicerae pilosula, put them in a round bottom flask, add 12 times the weight of distilled water to reflux for extraction, decoct for 1.5 hours for the first time, filter, add 10 times the amount of distilled water to reflux and extract for 1.5 hours, filter, Add 10 times the amount of distilled water to the filter residue, reflux and extract for 1 hour, combine the three extracts, concentrate and dry the extract under reduced pressure, and obtain a light yellow extract; dissolve the prepared light yellow extract in water, and apply the pretreated D101 type macroporous adsorption resin, combined with HPLC chromatographic analysis, to collect the part containing isochlorogenic acid; the elution conditions of the macroporous adsorption resin: the macroporous resin is treated with 5% NaOH, 10% acetic acid and 95% ethanol respectively before use, When the resin is washed with distilled w...
Embodiment 2
[0049] Example 2: Preparation of capsules of 3,5-dicaffeoylquinic acid methyl ester compound
[0050] prescription:
[0051] 3,5-DCQAME 15g
[0052] PVPK 30 1.5g
[0053] Microcrystalline Cellulose 4.5g
[0054] Low-substituted hydroxypropyl cellulose 0.45g
[0055] Sodium carboxymethyl starch 0.45g
[0056] Magnesium Stearate Appropriate amount
[0057]
[0058] 100 capsules
[0059] Mix the above-mentioned pharmaceutical composition with auxiliary materials, add an appropriate amount of distilled water, stir and mix well, probe ultrasonically (4000r / min) for 5 minutes, and high-pressure milk (pressure 1000bar) for 10 laps to obtain a suspension; vacuum-dry and pass through an 80-mesh sieve , granulated with 70% ethanol, dried, packed into capsules, made into 100 capsules, and obtained.
experiment example 3
[0060] Experimental example 3: Anti-hepatitis virus effect of 3,5-DCQAME
[0061] Test drug: 3,5-DCQAME (by the compound prepared in Example 1, batch number: 20091115)
[0062] Reagents: DMEM (produced by Gibco, USA); fetal bovine serum (produced by Gibco, USA); G-418 (produced by Sigma, USA); HbeAg, HbsAg stationary phase radioimmunoassay kit (produced by China Isotope Corporation, North Immunoreagent Research Institute);
[0063] 2.2.15 Cell: In order to transfect the whole genome of HBV (Aayw subunit), human hepatoblastoma cell line that can secrete HbeAg, HbsAg, and HBV-DNA particles (constructed by Mount sinai Medical Center in the United States, Institute of Pharmaceutical Biotechnology, Chinese Academy of Medical Sciences Virus chamber subculture).
[0064] Experimental method: Inoculate 24-well culture plate with 100,000 2.2.15 cells per milliliter, 1ml per well, 37℃5%CO 2 Incubate for 24 hours, add 2-fold dilution of the test solution below the non-toxic concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com