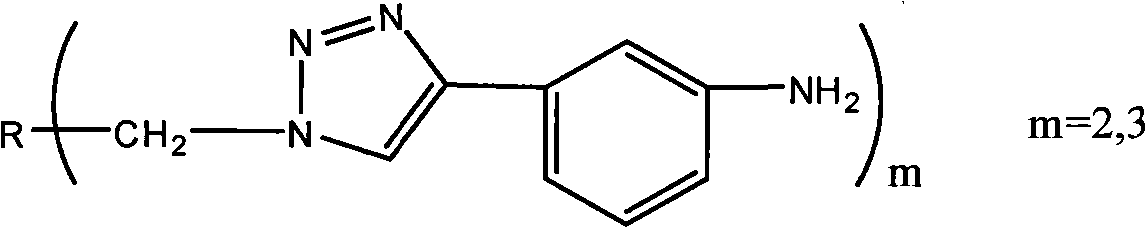
Polyamine containing triazole ring and preparation method and application thereof
A technology of polyamines and triazole rings, applied in the field of polyamines containing triazole rings and its preparation, can solve the problems of low yield, difficult synthesis, high cost, etc., and achieve excellent heat resistance and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
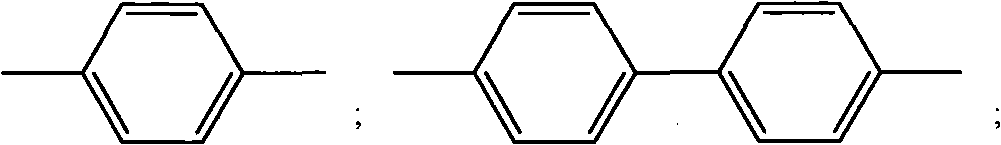
[0047] (1) Synthesis of 1,4-diazidemethylbenzene
[0048] Add p-dichloromethylbenzene (0.05mol), NaN 3 (0.15mol), toluene (20mL) and N,N-dimethylformamide (20mL), heated to 70-75°C under stirring, and reacted at constant temperature for 3 hours. After the reaction, the reaction product was cooled to room temperature, poured into 200mL of deionized water was left to stand overnight under freezing conditions to precipitate white flaky crystals, filtered, the filter cake was washed with deionized water, and dried to obtain a white powdery solid with a yield of 90%. Melting point: 27~29℃; FT-IR (KBr, v, cm -1 ): 2089 (-N 3 stretching vibration); 1 H-NMR (CDCl 3 , TMS) δ: 7.33 (s, 4H, Ha), 4.35 (s, 4H, Hb), its structural formula is:
[0049]
[0050] (2) Synthesis of 1,4-bis[4-(3-aminophenyl)-1,2,3-triazole-1-methylene]benzene
[0051] Add 1,4-diazidemethylbenzene (0.01mol), m-aminophenylacetylene (0.02mol), CuSO 4 ·5H 2 O (0.005mol), sodium ascorbate (0.01mol), N,N-dim...
Embodiment 2
[0054] (1) Synthesis of 4,4-diazidomethylbiphenyl
[0055] Add p-dichloromethylbiphenyl (0.05mol), NaN 3 (0.15mol), toluene (20mL) and DMF (20mL), heated to 70-75°C under stirring, and reacted at constant temperature for 3 hours. After the reaction was completed, the reaction product was cooled to room temperature, poured into 200mL deionized water, and stood overnight , a white solid was precipitated, filtered, the filter cake was washed with deionized water, and dried to obtain a white powdery solid with a yield of 89%. Melting point 68~71℃; FT-IR (KBr, v, cm -1 ): 2089 (-N 3 stretching vibration); 1 H-NMR (CDCl 3 , TMS) δ: 7.33 (s, 4H, Ha), 7.29 (s, 4H, Ha), 4.35 (s, 4H, Hb), its structural formula is:
[0056]
[0057] (2) Synthesis of 4,4-bis[4-(3-aminophenyl)-1,2,3-triazole-1-methylene]biphenyl
[0058] Add 1,4-diazidemethylbenzene (0.01mol), m-aminophenylacetylene (0.02mol), CuSO 4 ·5H 2 O (0.005 mol), sodium ascorbate (0.01 mol), triethylamine (0.02 mol), N,...
Embodiment 3
[0061] (1) Synthesis of ethylene glycol diazide
[0062] The synthesis reaction is carried out in two steps, first the chlorination of polyethylene glycol, and then azidation, the reaction equation is:
[0063]
[0064] In the 500mL flask equipped with stirring, add polyethylene glycol 0.08mol, SOCl 2 200mL, equipped with a spherical condenser and a gas absorption tube, heated to 65°C with an oil bath, and stirred for 72h. After the reaction was over, excess SOCl was distilled off under reduced pressure on a rotary evaporator. 2 , add NaHCO 3 The aqueous solution was adjusted to pH > 7, and filtered with suction. Filtrate with 50mL CHCl 3 Extract 3 times, then add anhydrous magnesium sulfate to dry, filter with suction, distill off the solvent to obtain the chlorinated product of polyethylene glycol.
[0065]In a 250mL single-necked round bottom flask equipped with stirring, add 0.08mol of the chlorinated product of polyethylene glycol synthesized above, NaN 3 0.4mol,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com