Stable protein kinase activator, preparation method thereof and use
A protein kinase and activator technology, applied in the field of medicine, to achieve the effect of easy storage and transportation, and stable storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
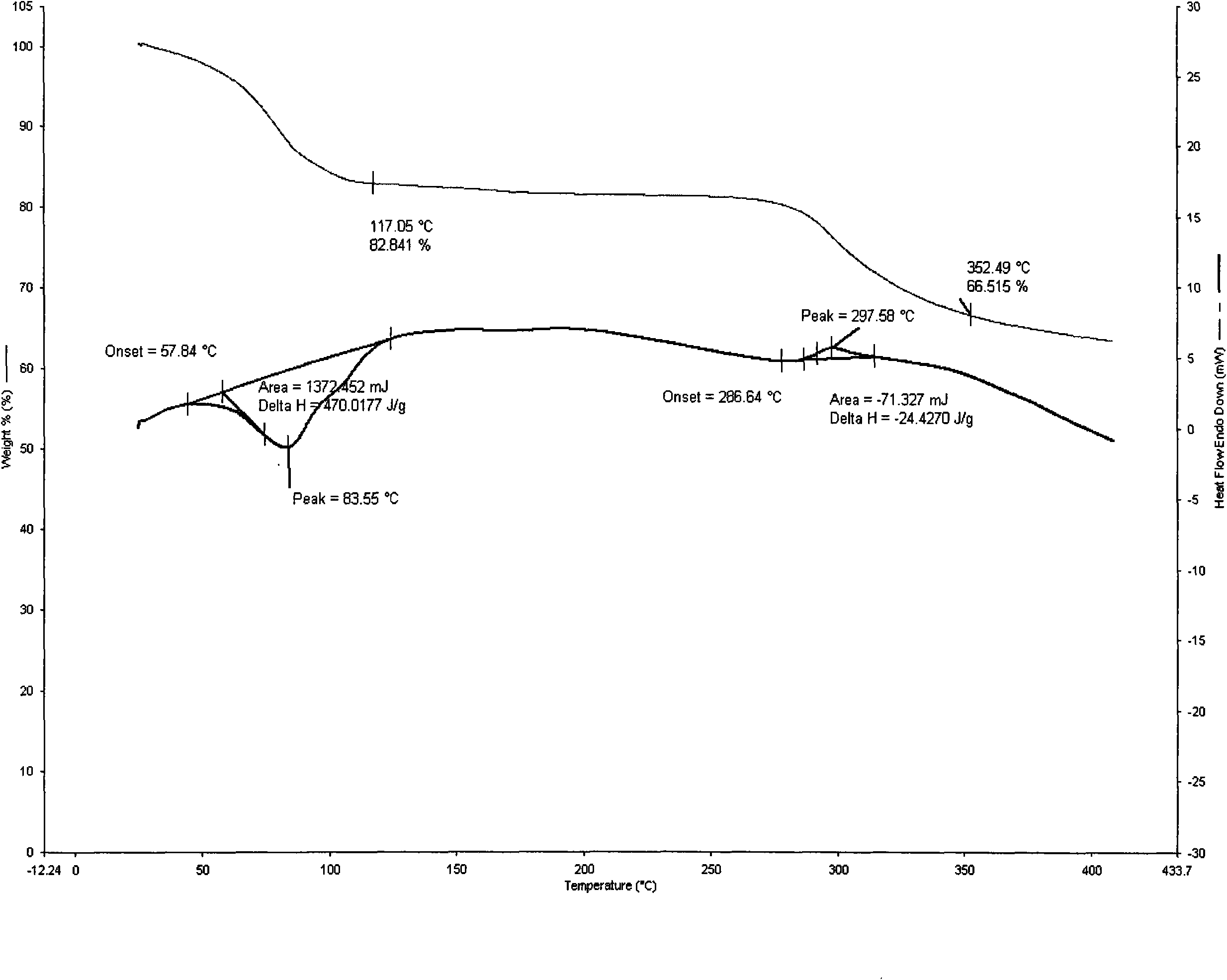
[0072] Example 1 Preparation of magnesium salt 8 hydrate of cyclic adenosine monophosphate In a 250ml three-necked flask, add cyclic adenosine monophosphate 0.1mol, water 100ml, stir, add 0.05mol magnesium oxide, stir at 20~60°C for 30~ Dissolve for 90 minutes, filter, add 1 to 5 times the amount of acetone to precipitate the solid, cool to -10 to 15°C, place it for 1 to 24 hours, filter, dry the solid at about 50°C for 2 to 4 hours to obtain off-white crystals Decomposition at 227°C (uncorrected), HPLC: cyclic adenosine monophosphate content 39.86%, water content measured by Karl Fischer method is 17.57%, thermal analysis: platform weight loss is about 17.16% (see attached figure 1 ), which is within the error range with the sample containing 8 crystal waters (theoretical value 17.48%). Elemental analysis theoretical value: C 29.12%, H4.64%, N16.98% P7.51%, Mg 2.95%; measured value: C 29.33%, H4.52%, N17.11% P7.67%, Mg 3.23 %.
Embodiment 2
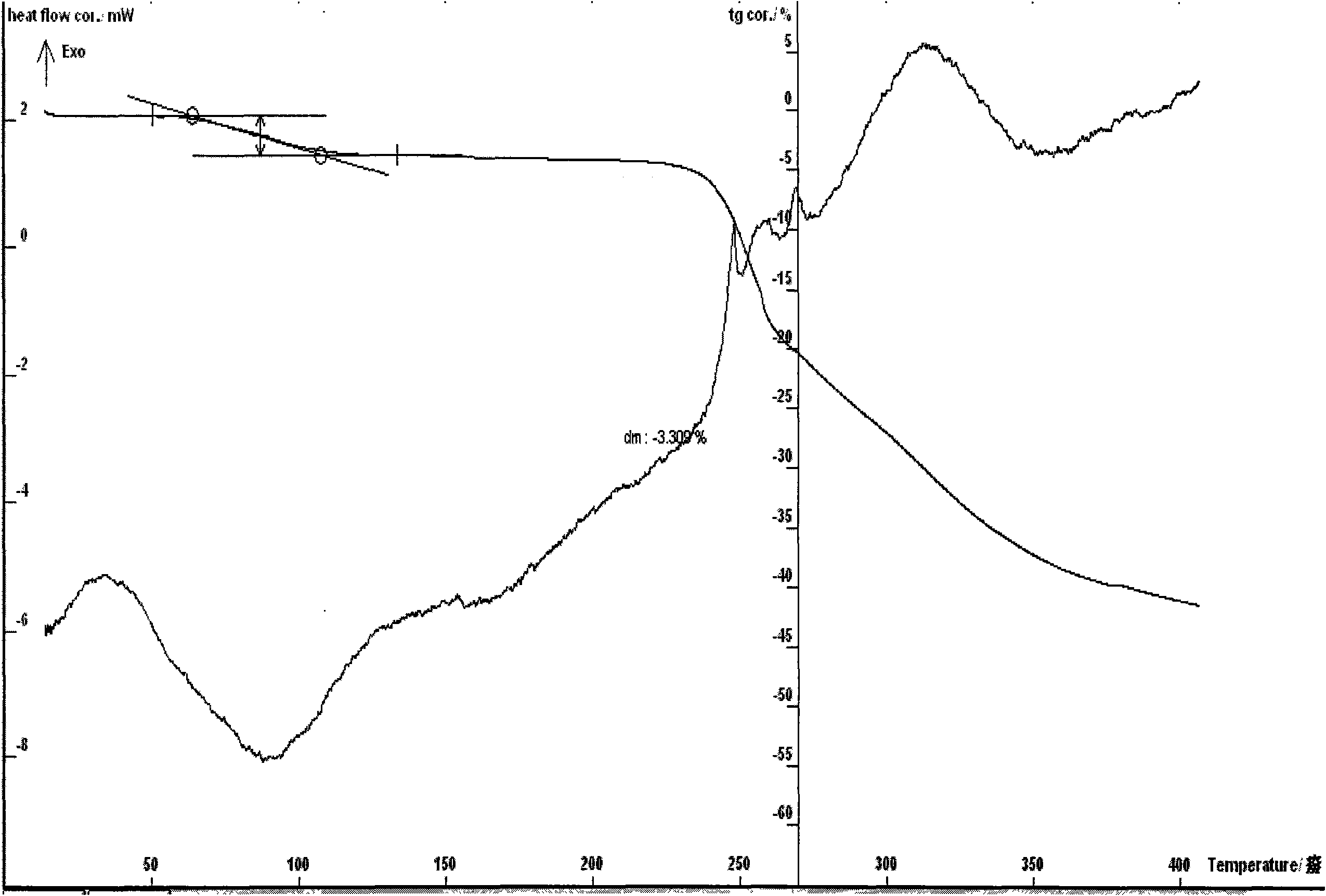
[0073] Example 2 Preparation of cyclic adenosine monophosphate calcium salt 3.5 hydrate In a 250ml three-necked flask, add 0.1mol of purified cyclic adenosine monophosphate, 100ml of water, stir, add 0.05mol of calcium hydroxide or calcium oxide, 20 Stir for 30 to 90 minutes at ~60°C to dissolve, filter, add 1 to 5 times the amount of absolute ethanol and isopropanol (1:1), cool to -10 to 15°C, and place for 1 to 24 hours. The solid was precipitated, filtered, and dried at about 50°C for 4-6 hours to obtain 19.2 grams of off-white solid, melting point: 223°C decomposition (ELECTROTHERMAL MELTING POINT APPARATUS, uncorrected), infrared spectrum: the moisture content was 8.52% as determined by the Karl Fischer method, and the thermal Analysis: The weight loss of the platform is about 8.4%, which is within the error range with the result of the sample containing 3.5 crystal waters (theoretical value 8.30%); elemental analysis theoretical value: C 31.63%, H3.85%, N18.44% P8.16 %, ...
Embodiment 3
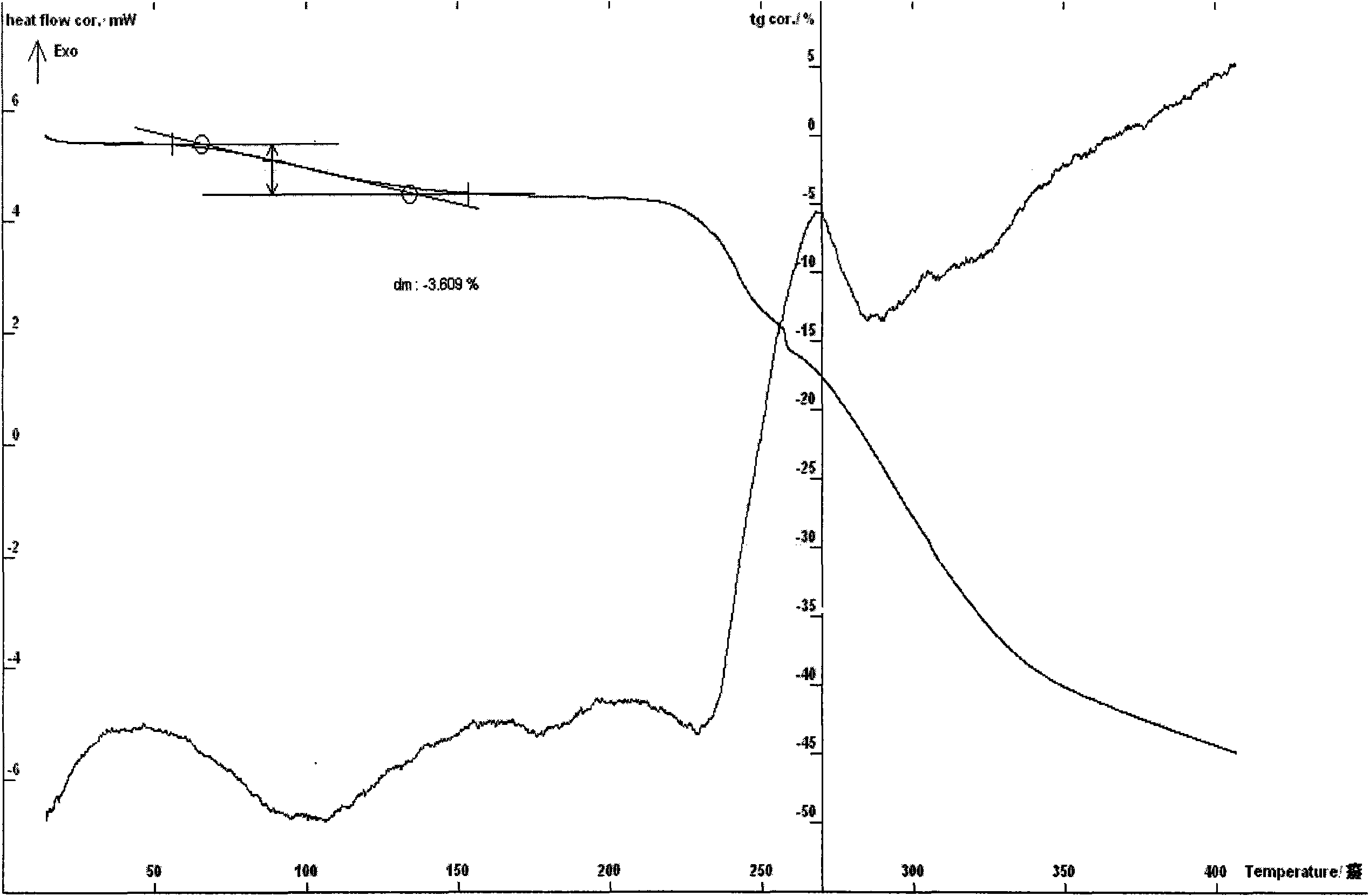
[0074]Example 3 Preparation of cyclic adenosine monophosphate meglumine monohydrate Put 10 g of purified cyclic adenosine monophosphate and equimolar meglumine into a reaction flask, add 200 ml of water, stir, and control the temperature between 10 and 60 ° C. For 10-120 minutes, add 1% of the total amount of the solution to the activated carbon and stir for half an hour to filter, then filter with a 0.22-micron microporous membrane or filter with an ultrafiltration membrane with a molecular weight cut-off of 6,000-30,000, and freeze it until -70~-30℃, keep it for about 24 hours, raise the temperature to about 20℃, dry it in vacuum, and then dry it at 20~30℃ for about 3~6 hours to get off-white crystalline powder, easily soluble in water, melting point: 67~ 71. ℃ (ELECTROTHERMAL MELTING POINT APPARATUS, uncorrected); HPLC: cyclic adenosine monophosphate content is 60.79%, ESI: m / z: 523; moisture (Karl Fischer method): 3.51%, thermal analysis: weight loss on the platform is abou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com