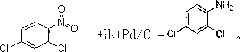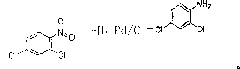Preparation method for synthesizing 2,4-dichloroaniline from 2,4-dichloronitrobenzene
A technology of dichloronitrobenzene and dichloroaniline, which is applied in the field of preparation of high-quality 2.4-dichloroaniline, can solve the problems of three wastes, poor product quality, and large equipment investment, and achieve simple process operation and equipment utilization rate High, good product quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] The reaction kettle used should be equipped with a stirring device, a thermometer, a condenser, a pressure gauge, and a gas venting device. First check whether the equipment is in good condition, then put into the reactor 25g of 2.4-dichloronitrobenzene with a weight percent content≥99%, 200ml of ethanol solvent, 1g of Pd / C catalyst and 0.2g of amino acid, and cover the lid of the reactor. Raise the temperature to 20°C-100°C, open the hydrogen valve, and start slowly feeding hydrogen at a pressure of 3-30MPa. The reaction temperature is controlled at 30-100°C, the pressure in the reactor is controlled at 4-25MPa, and hydrogen is passed for 6-16 hours. Under the condition of constant hydrogen flow rate, the pressure in the reactor began to increase slowly, indicating that the reaction was nearing the end. After taking a small sample to filter out the catalyst, evaporate the solvent in the small sample at normal temperature and pressure and perform liquid chromatography a...
Embodiment 2
[0015] The reaction kettle used should be equipped with a stirring device, a thermometer, a condenser, a pressure gauge, and a gas venting device. First check whether the equipment is in good condition, then put into the reactor 25g of 2.4-dichloronitrobenzene with a weight percent content≥99%, 200ml of propanol solvent, 1g of Pd / molecular sieve catalyst, and cover the reactor lid. Raise the temperature to 20°C-100°C, open the hydrogen valve, and start slowly feeding hydrogen at a pressure of 3-30MPa. The reaction temperature is controlled at 30-100°C, the pressure in the reactor is controlled at 4-25MPa, and hydrogen is passed for 6-16 hours. Under the condition of constant hydrogen flow rate, the pressure in the reactor began to increase slowly, indicating that the reaction was nearing the end. After taking a small sample to filter out the catalyst, evaporate the solvent in the small sample at normal temperature and pressure and perform liquid chromatography analysis. When t...
Embodiment 3
[0017] The reaction kettle used should be equipped with a stirring device, a thermometer, a condenser, a pressure gauge, and a gas venting device. First check whether the equipment is in good condition, then drop into the reactor 25g of 2.4-dichloronitrobenzene with a weight percent content≥99%, 200ml of methanol solvent, 1g of Raney nickel catalyst and 0.2g of amino acid, and cover the lid of the reactor. Raise the temperature to 20°C-100°C, open the hydrogen valve, and start slowly feeding hydrogen at a pressure of 3-30MPa. The reaction temperature is controlled at 30-100°C, the pressure in the reactor is controlled at 4-25MPa, and hydrogen is passed for 6-16 hours. Under the condition of constant hydrogen flow rate, the pressure in the reactor began to increase slowly, indicating that the reaction was nearing the end. After taking a small sample to filter out the catalyst, evaporate the solvent in the small sample at normal temperature and pressure and perform liquid chroma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com