Compound tylosin injection for animals and preparation method thereof
A technology of tylosin and injection, applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, antibacterial drugs, etc., can solve the problem of incomplete curative effect, achieve long action time, short course of treatment, and stimulating effect small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A compound tylosin injection for veterinary use, the components are as follows:
[0040] Tylosin 10g, doxycycline hydrochloride 5g, trimethoprim 3.75g, aminophylline 4g, dexamethasone sodium phosphate 0.05g, magnesium chloride 2.35g, sodium formaldehyde sulfoxylate 0.25g, sodium thiosulfate 0.15g, α-pyrrolidone 30g, N,N-dimethylformamide 15g, propylene glycol 15g, water for injection 20g.
[0041] The above-mentioned veterinary compound tylosin injection is prepared by the following steps:
[0042] 1) After dissolving 2.35 g of magnesium chloride in 3 g of water for injection, add 30 g of α-pyrrolidone, then add 5 g of doxycycline hydrochloride, stir and dissolve to obtain doxycycline hydrochloride solution;
[0043] 2) After dissolving 0.25 g of sodium formaldehyde sulfoxylate in 3 g of water for injection, add it to the solution prepared in step 1), then add 2 g of ethanolamine and stir evenly, then add 10 g of tylosin, stir to dissolve, and obtain Tylosin mixed sol...
Embodiment 2
[0067] A compound tylosin injection for veterinary use, the components are as follows:
[0068] Tylosin 5g, doxycycline hydrochloride 3g, Shenoxybenz 2g, aminophylline 3g, dexamethasone sodium phosphate 0.02g, magnesium chloride 1.41g, sodium formaldehyde sulfoxylate 0.1g, sodium thiosulfate 0.1 g, α-pyrrolidone 20g, N,N-dimethylformamide 20g, propylene glycol 20g; water for injection 30g;
[0069] The preparation steps were the same as in Example 1 to obtain compound tylosin injection for veterinary use, with a pH of 8.5.
[0070] The present embodiment clinical treatment effect is as follows:
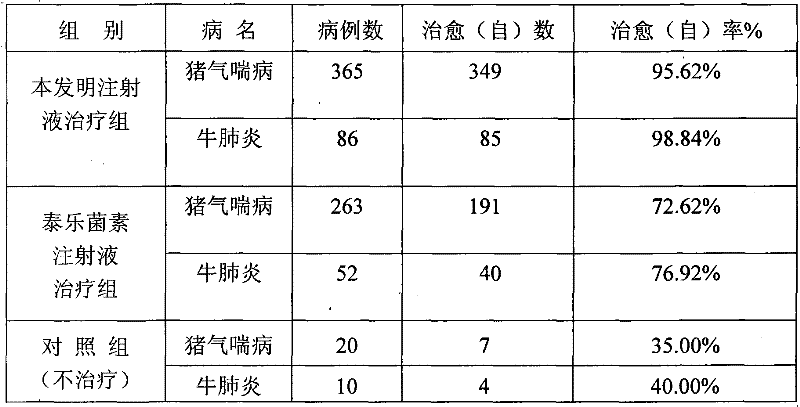
[0071] Table 1 Clinical therapeutic effect of compound tylosin injection
[0072] animal
[0073] Table 2 The present invention and tylosin injection clinical treatment comparative test result
[0074]
[0075] The stability test of product is with embodiment 1.
[0076] when saving
[0077] From the above data, it can be seen that after 3 months of acceler...
Embodiment 3
[0079] A compound tylosin injection for veterinary use, the components are as follows:
[0080] Tylosin 15g, doxycycline hydrochloride 7g, trimethoprim 3g, aminophylline 3g, dexamethasone sodium phosphate 0.1g, magnesium chloride 3.3g, sodium formaldehyde sulfoxylate 0.25g, sodium thiosulfate 0.15 g, α-pyrrolidone 40g, N,N-dimethylformamide 15g, propylene glycol 15g; water for injection 20g;
[0081] The preparation steps were the same as in Example 1 to obtain compound tylosin injection for veterinary use, with a pH of 8.7.
[0082] The present embodiment clinical treatment effect is as follows:
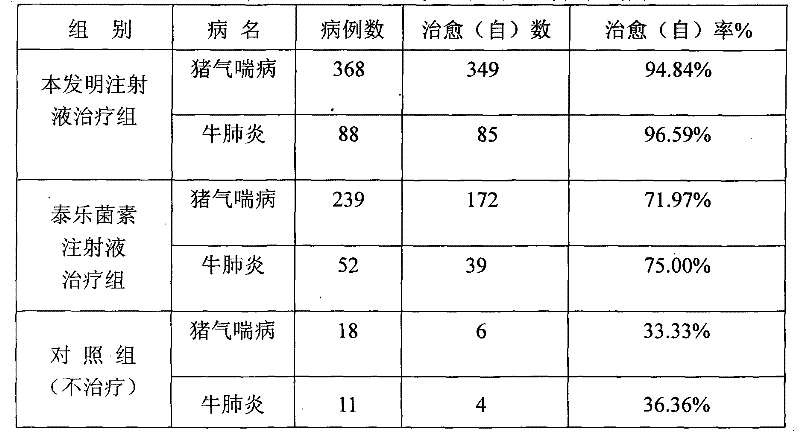
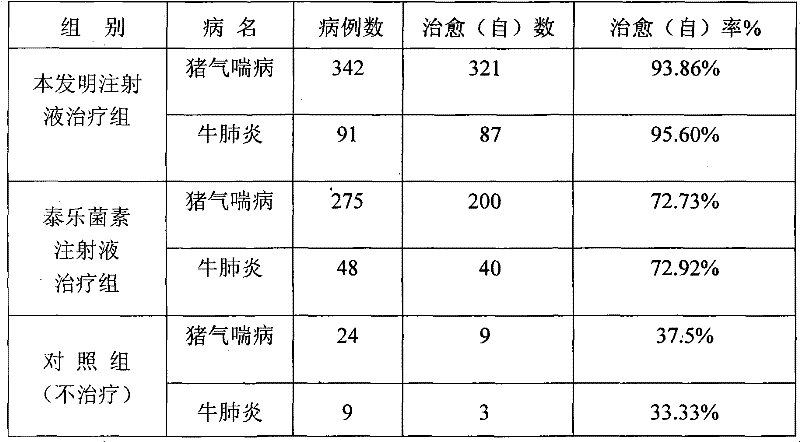
[0083] Table 1 Clinical therapeutic effect of compound tylosin injection
[0084] animal
[0085] Table 2 The present invention and tylosin injection clinical treatment comparative test result
[0086]
[0087] The stability test of product is with embodiment 1.
[0088]
[0089] It can be seen from the above data that after 3 months of accelerated prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com