Application of diterpene ginkgolide
A diterpene lactone and compound technology, applied in the field of diterpene lactone compounds, can solve the problems of the JAK-STAT signaling pathway of Euphorbia chamaejasma extract that has not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
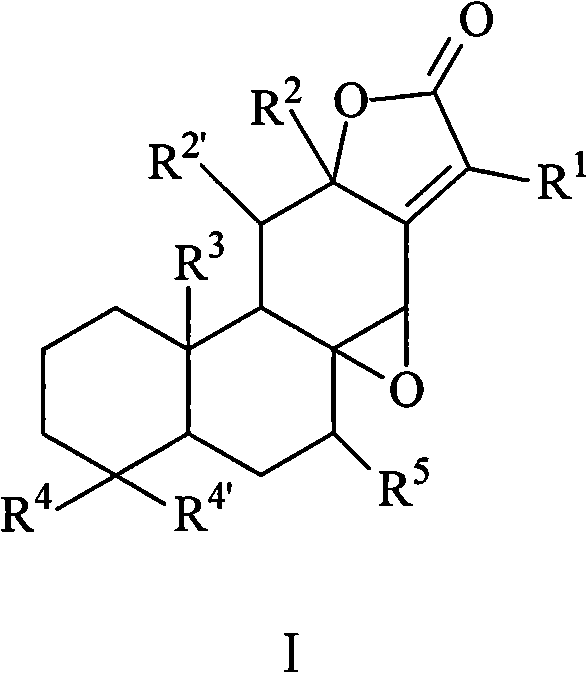
[0086] Embodiment 1 compound preparation
[0087] Extraction and separation of Euphorbia chamaejasme:
[0088] 10Kg root of Euphorbia fishceriana Steud. purchased from Shanghai Xujiahui Traditional Chinese Medicine Decoction Pieces Factory was extracted three times (50L×3) with 95% industrial alcohol at room temperature, the extracts were combined, concentrated under reduced pressure to remove the solvent to obtain ethanol extract. Disperse the ethanol extract in hot water and extract with petroleum ether (5L×3) and chloroform (5L×3) respectively to obtain 300g of petroleum ether extract and 883g of chloroform extract. For the chloroform part, the normal phase silica gel column [elution system is petroleum ether-acetone, petroleum ether-ethyl acetate, cyclohexane-acetone], gel column LH-20 [elution system chloroform-methanol=5:1] and Reverse-phase silica gel column (Rp-18) [elution system is methanol-water] for separation, crude crystals were purified by crystallization metho...
Embodiment 217
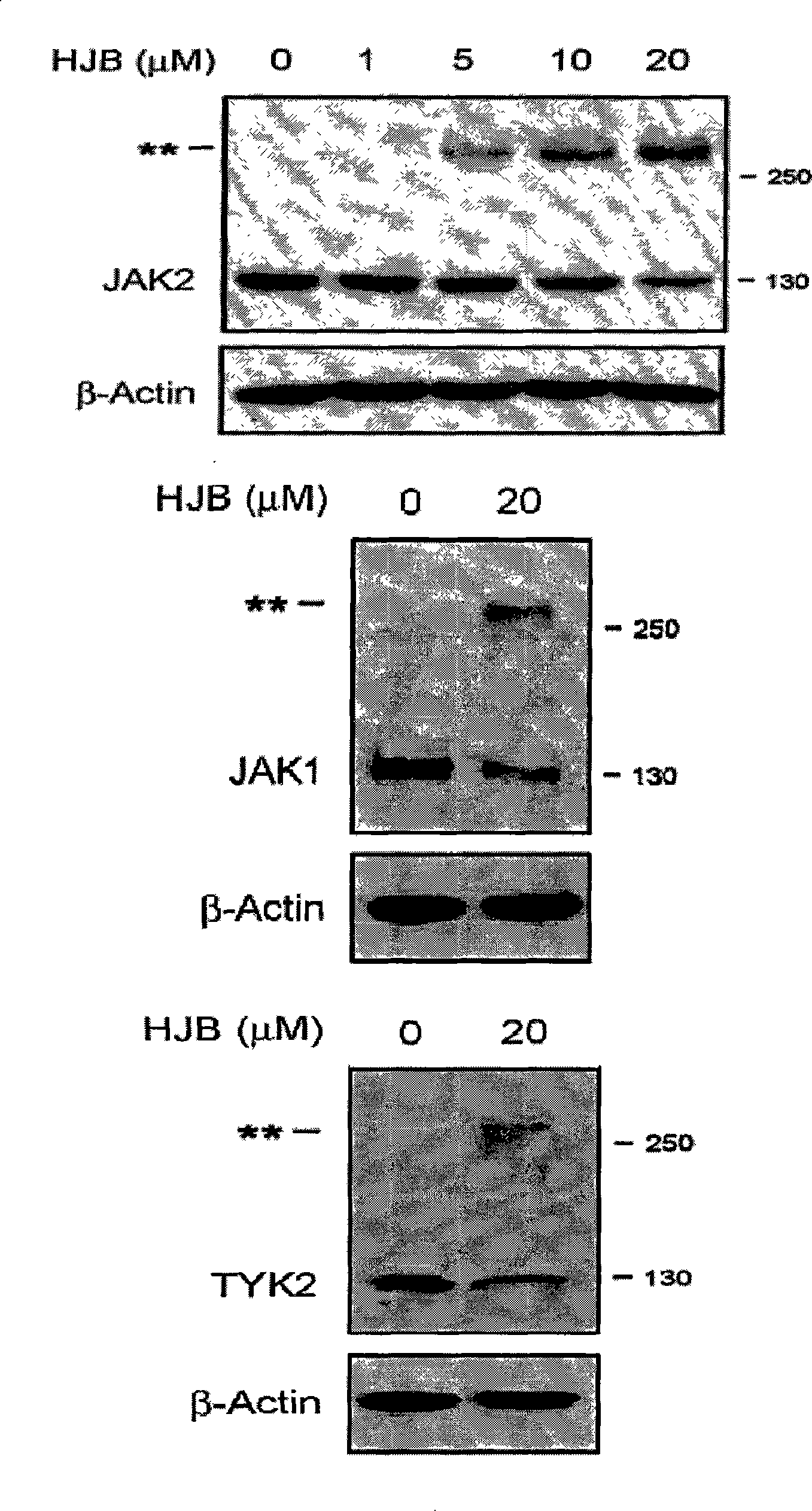
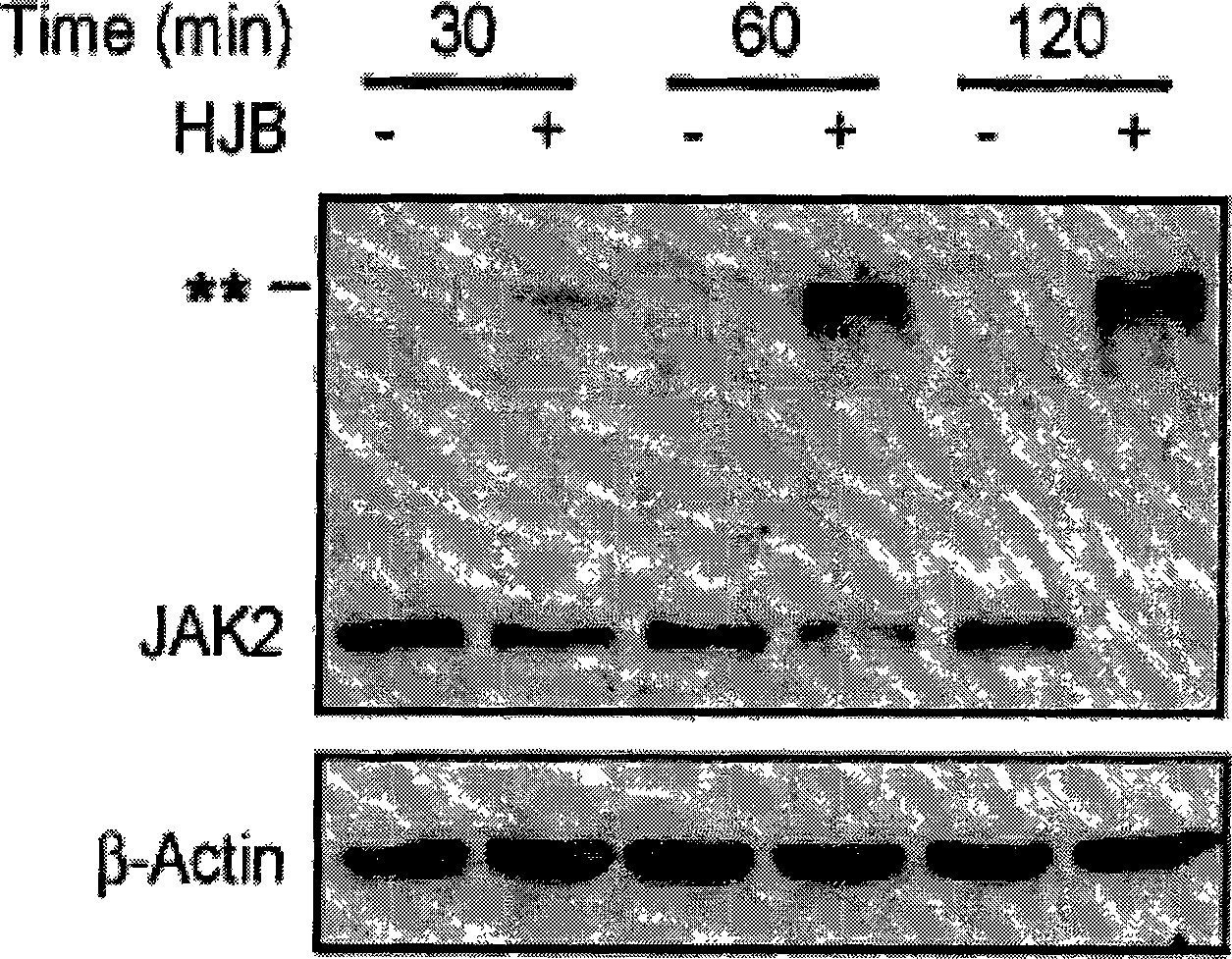
[0092] Example 217-hydroxy-jolkinolide B inhibits STAT3 signaling pathway
[0093] Experimental Materials:
[0094] 1) Cell line: HepG2 cells stably transfected with STAT3-luciferase reporter gene plasmid.
[0095] 2) IL-6 (purchased from Peprotech Asia)
[0096] 3) Drug to be tested: 17-hydroxy-jolkinolide B, prepared in Example 1;
[0097] 4) Luciferase detection kit (purchased from Promega)
[0098] experimental method:
[0099] 1) HepG2 cells in good growth state, digested with trypsin, resuspended the cells in αMEM medium and adjusted the cell concentration to 2×105 / ml, took 100 μl of the cell suspension and seeded it in a 96-well plate, and incubated at 37°C, 5% CO2 Cultivate in the incubator for 48 hours until the cells are completely attached to the wall;
[0100] 2) When the cells grow to a density of 60-70%, they are randomly divided into the following 3 groups:
[0101] a. Negative control group, without IL-6 and test drug;
[0102] b. IL-6 group, add IL-6 to...
Embodiment 3
[0112] Inhibitory effect on the growth of various tumor cells in the in vitro test of embodiment 3
[0113] In vitro culture of human embryonic kidney cancer 293 cells, human liver cancer HepG2 cells, human breast cancer MDA-MB-468, MDA-MB-231, MDA-MB-453, MCF-7 cells, human gastric cancer HGC cells, human lymphoma U937 cells , Human cervical cancer Hela cells. After the cells grow to the logarithmic growth phase, digest the cells with trypsin, centrifuge at 1000rpm for 5 minutes, discard the supernatant, suspend with an appropriate amount of medium, and adjust the cell concentration to 3.5 104 / ml. The cell suspension was inoculated into a 96-well cell culture plate, 100 μl per well, placed in a cell culture incubator (37° C., 5%) and cultured for 24 hours. Each medication group was added with 17-hydroxy-jolkinolide B diluted in cell culture medium, respectively. 100 μl of 17-hydroxyjolkinolide B, 17-hydroxyjolkinolide B acetic acid ester, and 17-hydroxyjolkinolide Bbenzoic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com