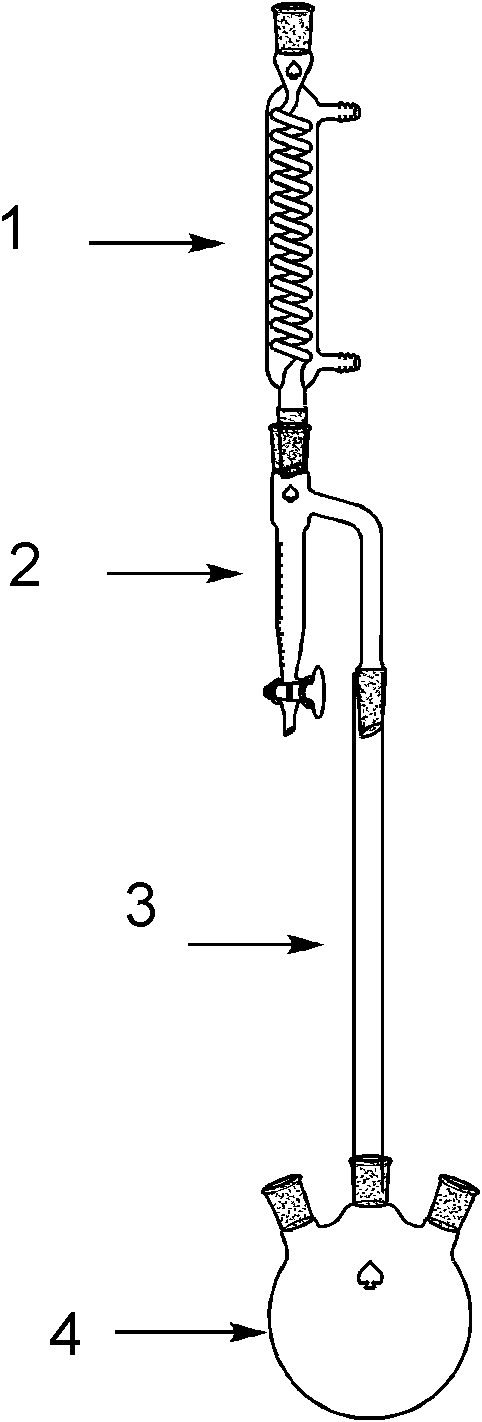
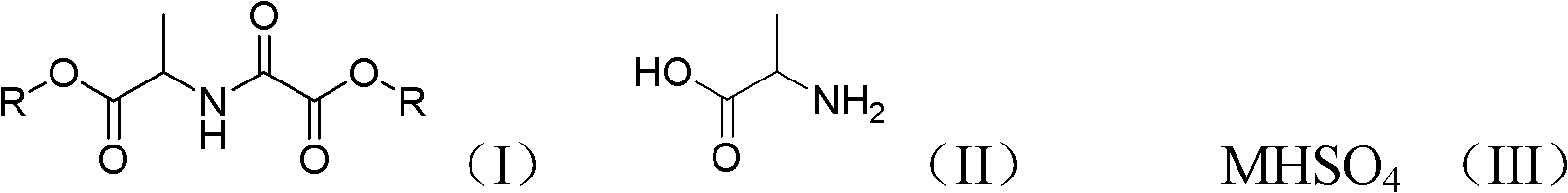
Chemical synthesis method of N-alcoxyloxalyl alanine ester
A kind of technology of alkoxy oxalylalanine ester and synthesis method, which is applied in chemical instruments and methods, organic chemistry, carboxylic acid amide preparation, etc., can solve the problems of harsh reaction conditions, low yield, difficult separation, etc., and achieve the goal of reaction The effect of mild conditions, advanced process route, and fast esterification speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 89g (1mol) of alanine, 180g (2mol) of oxalic acid, and 460g of ethanol into a 2L four-neck flask equipped with a thermometer, a rectifying column, a water separator, a reflux condenser, and mechanical stirring, heat to dissolve, and add hydrogen sulfate Sodium 6g (0.05mol), with water agent toluene 200g, heated to reflux, rectified water for 40 hours. After the reaction is complete, add 50 mL of water, separate the water layer, dry the organic phase with 50 g of anhydrous sodium sulfate, evaporate the solvent at normal pressure until the internal temperature reaches 120 ° C, and recover the oxalyl diester under reduced pressure at 2 mmHg below 120 ° C to obtain light yellow Liquid N-ethoxyoxalylalanine ethyl ester 198.7g, gas chromatography detection content 95%, yield 87%, MS (EI): 217.3.
Embodiment 2
[0027] In a 2L four-neck flask equipped with a thermometer, rectifying column, water separator, reflux condenser and mechanical stirring, add 89g (1mol) of alanine, 180g (2mol) of oxalic acid, 600g of n-propanol, heat to dissolve, add 6 g (0.05 mol) of sodium bisulfate, 100 g of n-hexane with water agent, the temperature was raised to reflux, and the water was distilled for 40 hours. After the reaction is complete, add 50 mL of water, separate the water layer, dry the organic phase with 50 g of anhydrous sodium sulfate, evaporate the solvent at normal pressure until the internal temperature reaches 120 ° C, and recover the oxalyl diester under reduced pressure at 2 mmHg below 120 ° C to obtain light yellow Liquid n-propyl N-propoxyoxalylalanine 213.7g, content 94%, yield 82%, MS (EI): 245.2.
Embodiment 3
[0029] In a 2L four-neck flask equipped with a thermometer, rectifying column, water separator, reflux condenser and mechanical stirring, add 89g (1mol) of alanine, 180g (2mol) of oxalic acid, 740g of n-butanol, heat to dissolve, add Sodium bisulfate 6g (0.05mol), water-carrying benzene 200g, warm up to reflux, rectify water for 40 hours. After the reaction is completed, add 50 mL of water, separate the water layer, dry 50 g of the organic phase with anhydrous sodium sulfate, evaporate the solvent at normal pressure until the internal temperature reaches 120 ° C, and recover the oxalyl diester under reduced pressure at 2 mmHg below 120 ° C to obtain light yellow Liquid n-butyl N-butoxyoxalylalanine 246.9g, content 94%, yield 85%, MS (EI): 273.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com