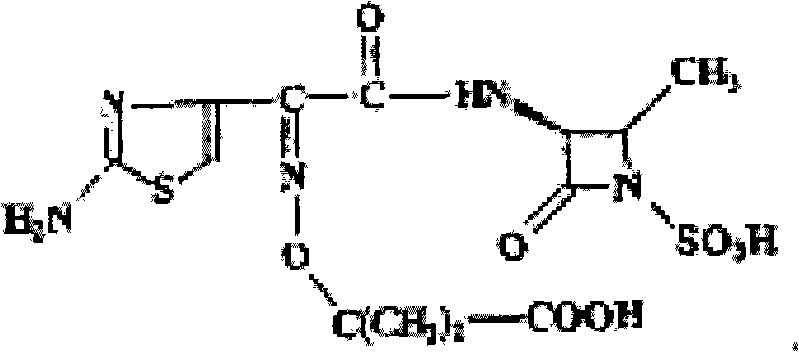
Aztreonam anhydrous crystal compound and preparation method thereof
An anhydrous crystalline compound technology, applied in the field of aztreonam anhydrous crystalline compound and its preparation, can solve the problems of poor antibacterial effect and no antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 : Preparation of aztreonam anhydrous crystal compound
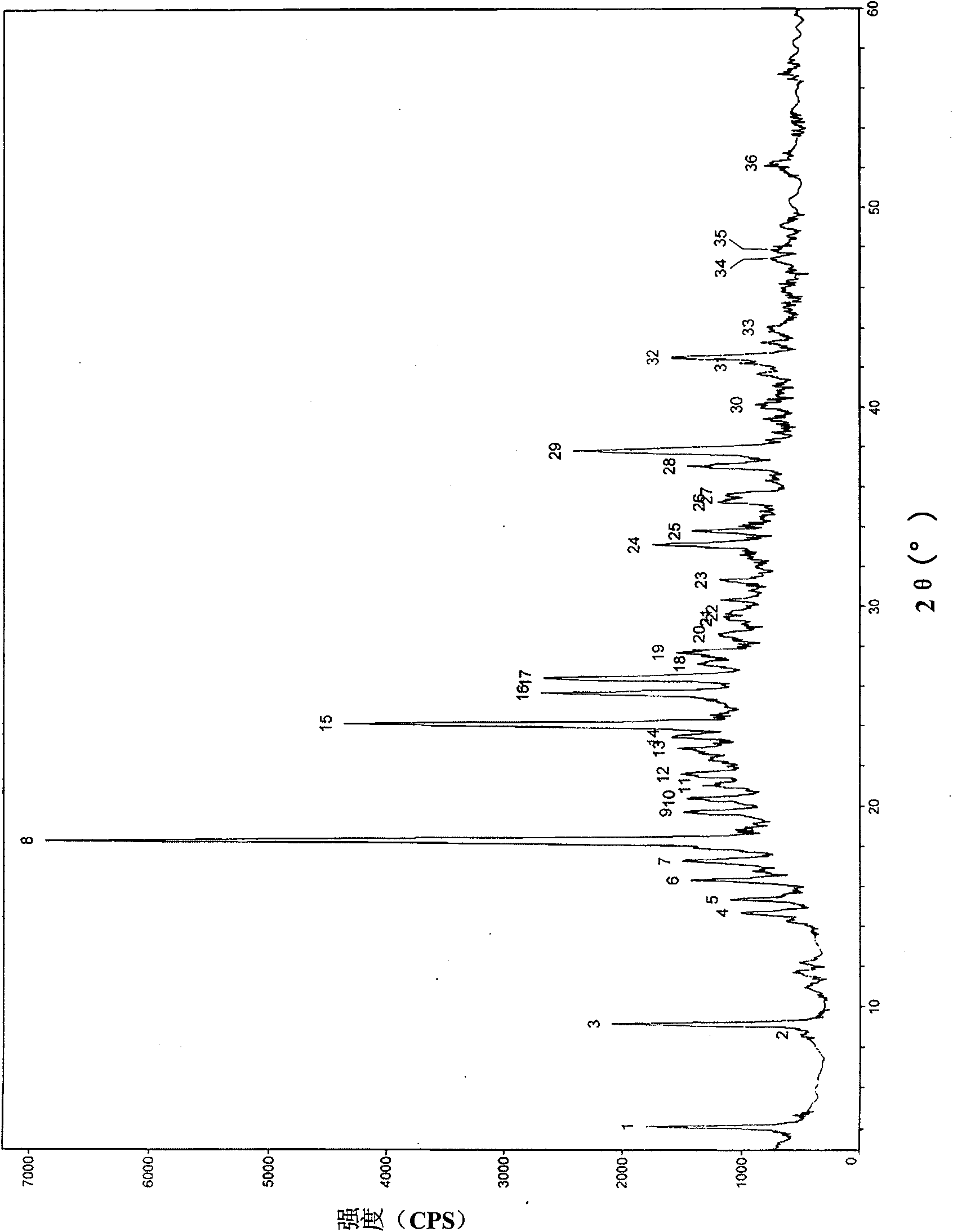
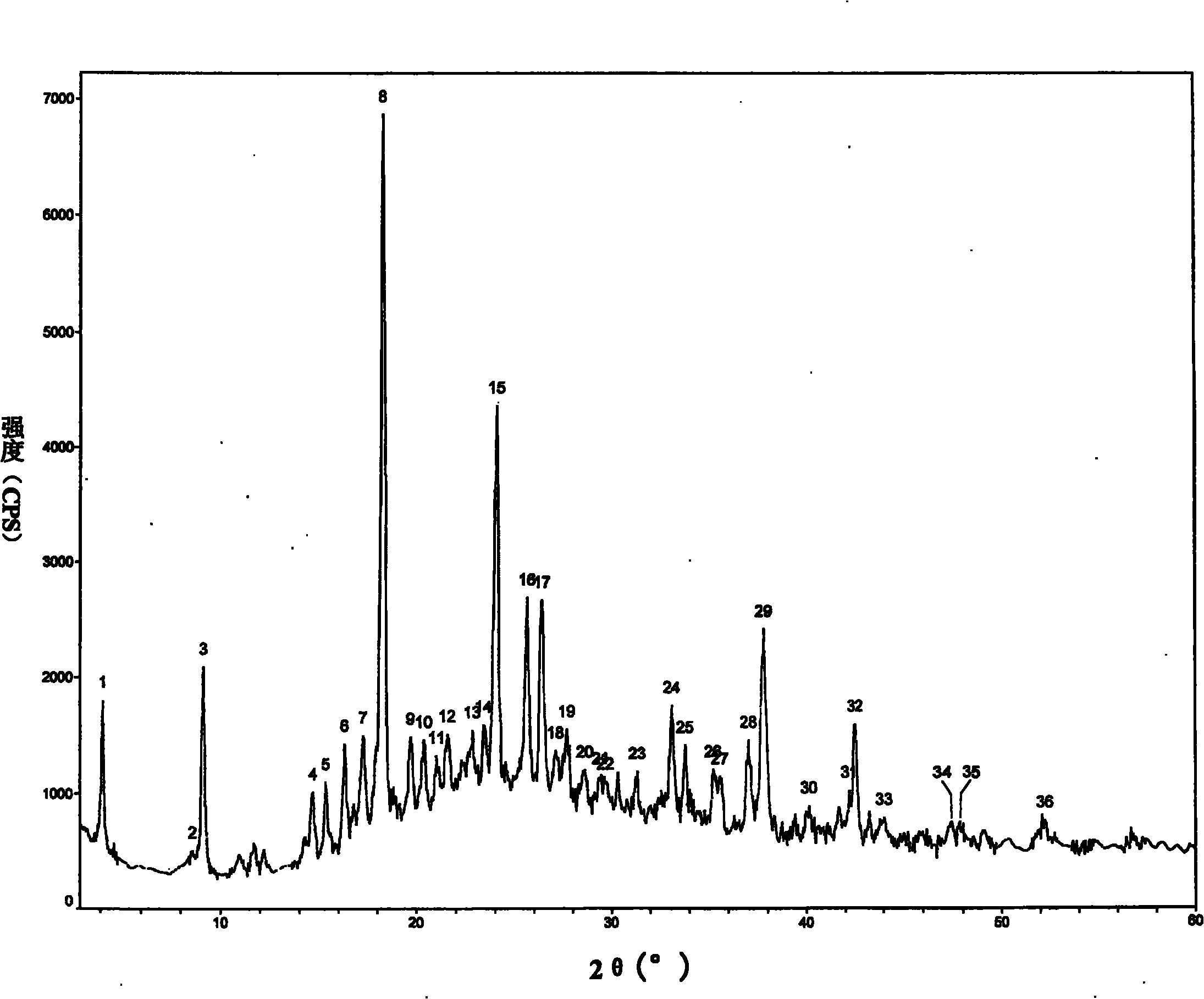
[0033] Add 1 g of aztreonam into 5 mL of anhydrous acetonitrile, reflux and dissolve at 80° C. for 1 h, and stir and cool the filtrate at room temperature for 6 h to crystallize. Suction filtration was then performed, and the filter cake was washed with a small amount of anhydrous acetonitrile. The filter cake was vacuum dried at 60°C to constant weight. Obtained 0.80 g of a crystal compound sample.
[0034] Use the RigakuD / MAXRC diffractometer to measure the prepared crystalline compound samples. The system parameters are as follows: CuK 1 Wire Monochromatic radiation, 40kV, 200mA. The obtained X-ray powder diffraction pattern is shown in figure 1 .
Embodiment 2
[0035] Example 2 : Performance identification of aztreonam anhydrous crystal compound
[0036] Under laboratory conditions, the fluidity (measurement of the angle of repose) of the aztreonam anhydrous crystal compound powder prepared in Example 1 was detected.
[0037] Basis: The angle of repose was measured by the fixed funnel method to investigate the fluidity of the anhydrous crystal compound of aztreonam.
[0038] Method: Gently and evenly drop the predicted material into the center of the disc to make the powder form a cone, stop feeding when the material falls freely from the inclined edge of the powder along the edge of the disc, and measure the angle of repose with a protractor (or Measure the radius of the disc and the height of the powder, and calculate the angle of repose, tgθ=height / radius). The smaller the angle of repose, the better the fluidity of the aztreonam anhydrous crystal compound.
[0039] Results: According to the above method, the measurement resul...
Embodiment 3
[0047] Example 3 : Preparation of aztreonam freeze-dried powder injection
[0048] The 24.2Kg aztreonam anhydrous crystal compound prepared by the method of Example 1 is mixed with 16.6Kg L-arginine powder, and 170.0Kg of water for injection is added under stirring and mixed until the powder is completely dissolved, and 1638.5g L-arginine is added to the solution. Arginine, adjust the pH of the solution to 5, and then add water for injection to adjust the final volume to 220L. The solution passes through a clarification filter and a sterile filter into a sterile tank. An aliquot of the resulting solution is poured into vials to obtain the following vial-filled medicinal solution:
[0049] Flushing volume Aztreonam potency / vial
[0050] 5mL 0.55g
[0051] 10mL 1.1g
[0052] 20mL 2.2g
[0053] A partially opened stopper with a catheter is placed over the vial and the contents are lyophilized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com