Micro-spherical polymer solid acid esterification catalyst and preparation method thereof
A polymer, esterification technology, applied in the preparation of carboxylate, catalysts for physical/chemical processes, chemical instruments and methods, etc., can solve the difficulty of product purification and raw material recovery, low specific activity of solid acid catalysts, hindering resin It can easily control the size of the microspheres, improve the catalytic selectivity, and increase the reaction speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
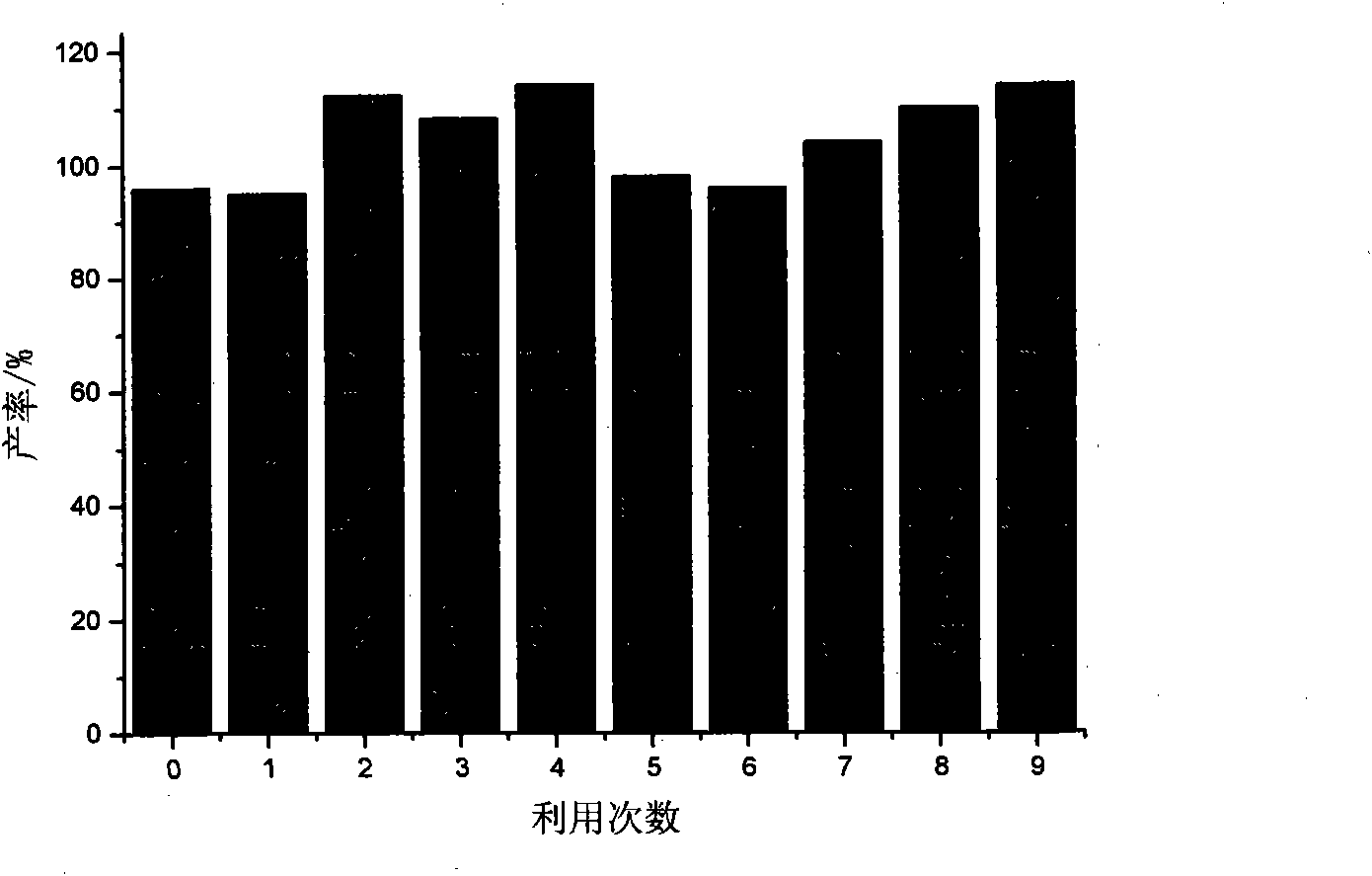
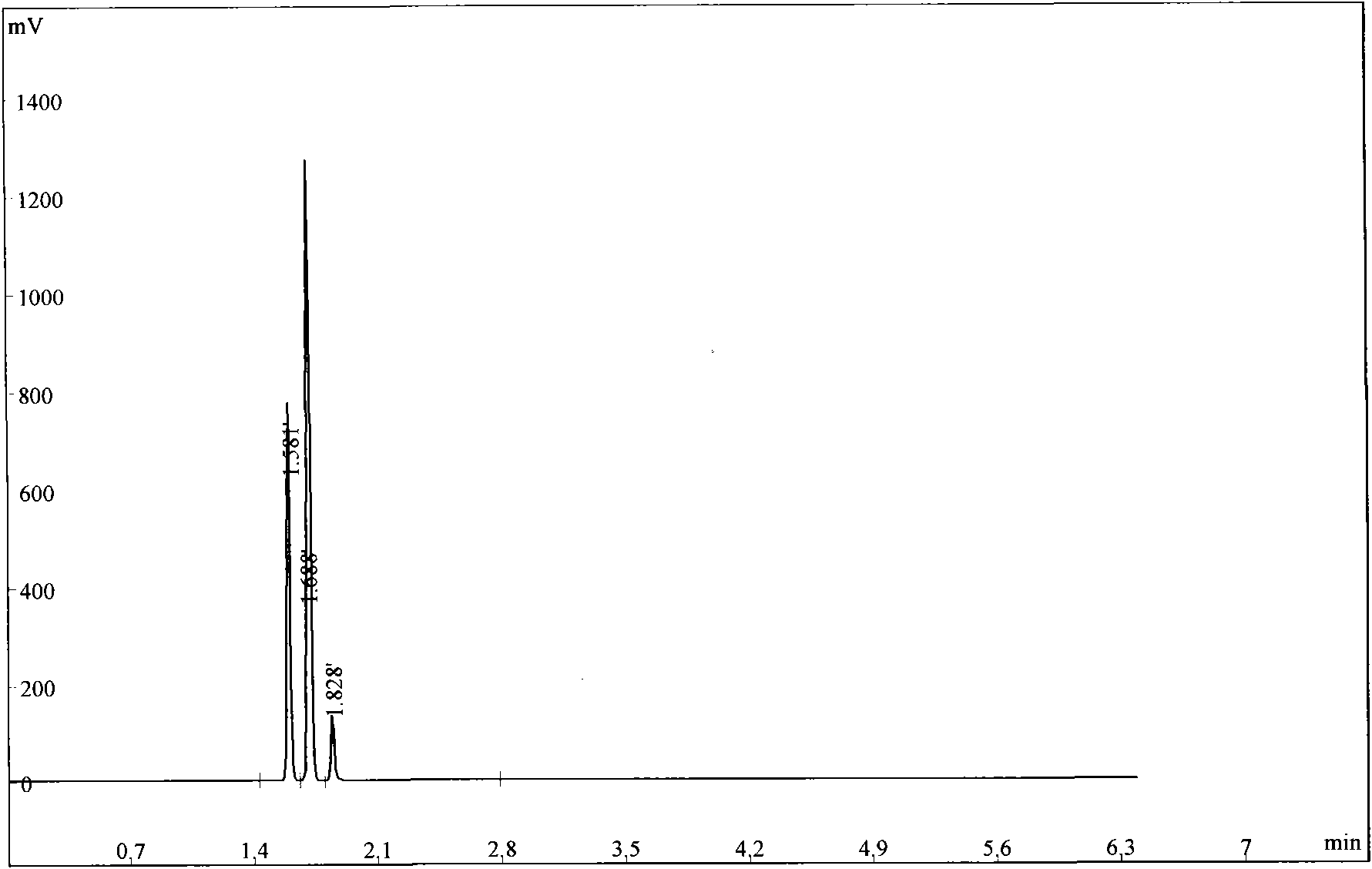
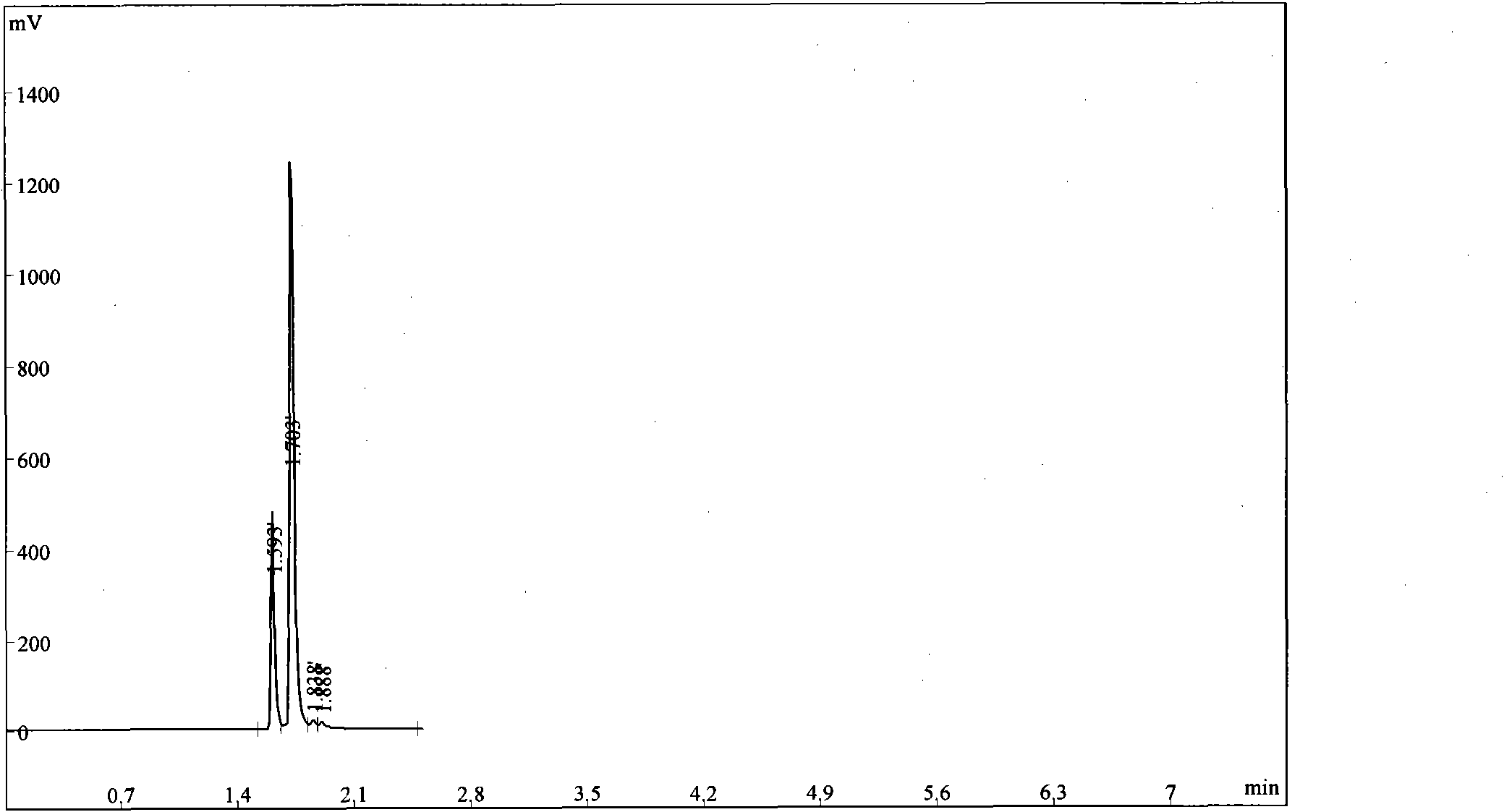
Image
Examples
Embodiment 1
[0050] Add 100 parts by mass of distilled water to the reactor, under stirring, add 30 parts by mass of styrene and sodium propylene sulfonate (the molar ratio of styrene and sodium propylene sulfonate is 1:2), 1 part by mass of potassium persulfate / sulfuric acid 0.3 parts by mass of sodium hydrogen, 2 parts by mass of sodium dodecylbenzenesulfonate; react for 4 hours in a boiling state to form an emulsion, cool and acidify the reactant to obtain a white solid precipitate, and dry in a vacuum oven to obtain a microspherical polymer solid acid catalyst.
Embodiment 2
[0052] Add 100 parts by mass of distilled water to the reaction kettle, under stirring, add a total of 30 parts by mass of acrylonitrile and sodium propylene sulfonate (the molar ratio of acrylonitrile and sodium propylene sulfonate is 1:2), 1 part by mass of potassium persulfate, hydrogen sulfate 0.3 parts by mass of sodium, 2 parts by mass of sodium dodecylbenzenesulfonate; reacted for 4 hours in a boiling state to form an emulsion, cooled and acidified the reactant to obtain a white solid precipitate, dried in a vacuum oven, and obtained a microspherical polymer solid acid catalyst .
Embodiment 3
[0054] Add 100 mass parts of distilled water in the reaction kettle, under stirring, add styrene, acrylonitrile and sodium propylene sulfonate (the molar ratio of styrene, acrylonitrile and sodium propylene sulfonate is 0.5:0.5:2) totally 30 mass parts, high 1 mass part of potassium manganate, 0.2 mass part of oxalic acid, and 2 mass parts of sodium dodecylbenzenesulfonate; react in a boiling state for 3 hours to form an emulsion, cool and acidify the reactant to obtain a white solid precipitate, dry in a vacuum oven, and obtain micro Spherical polymer solid acid catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com