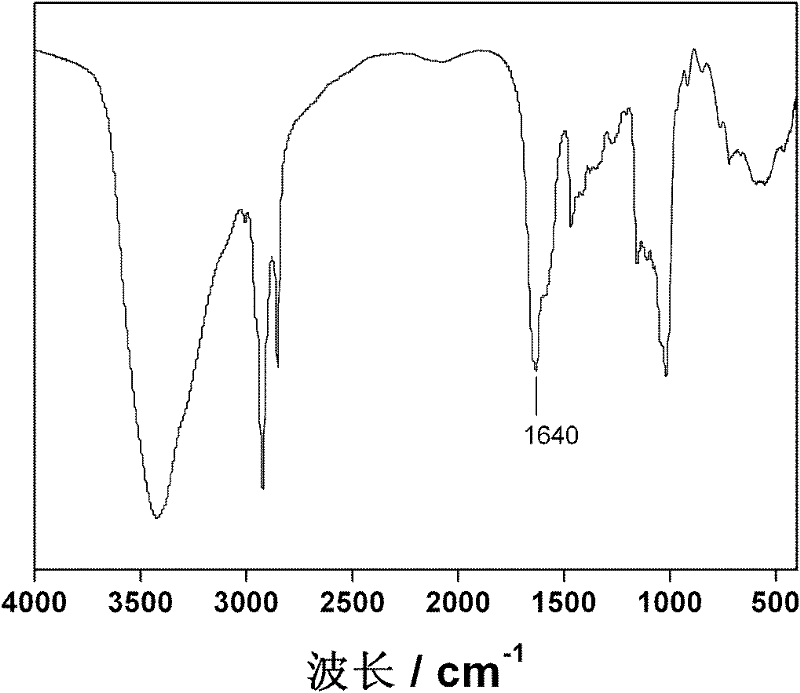
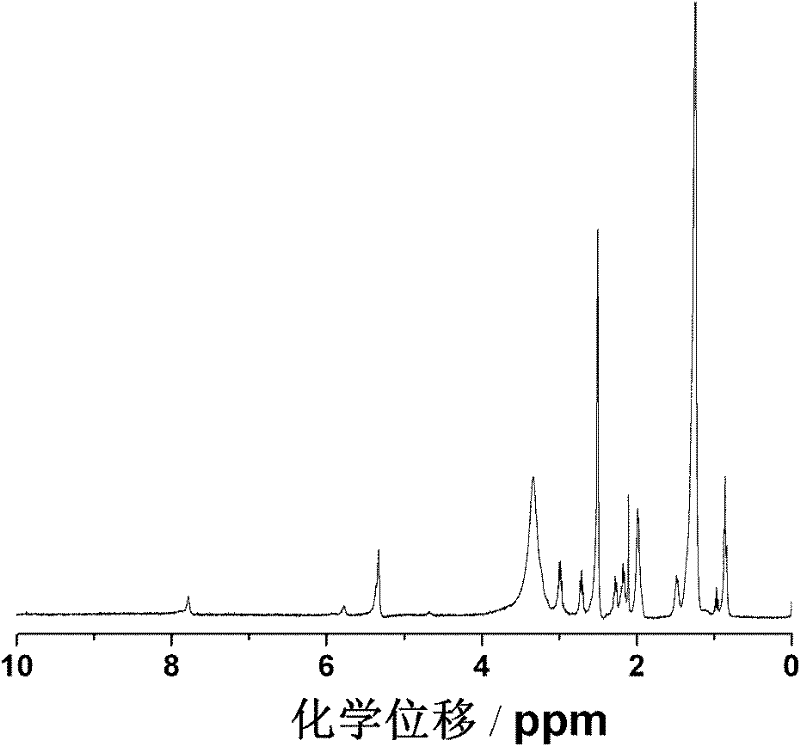
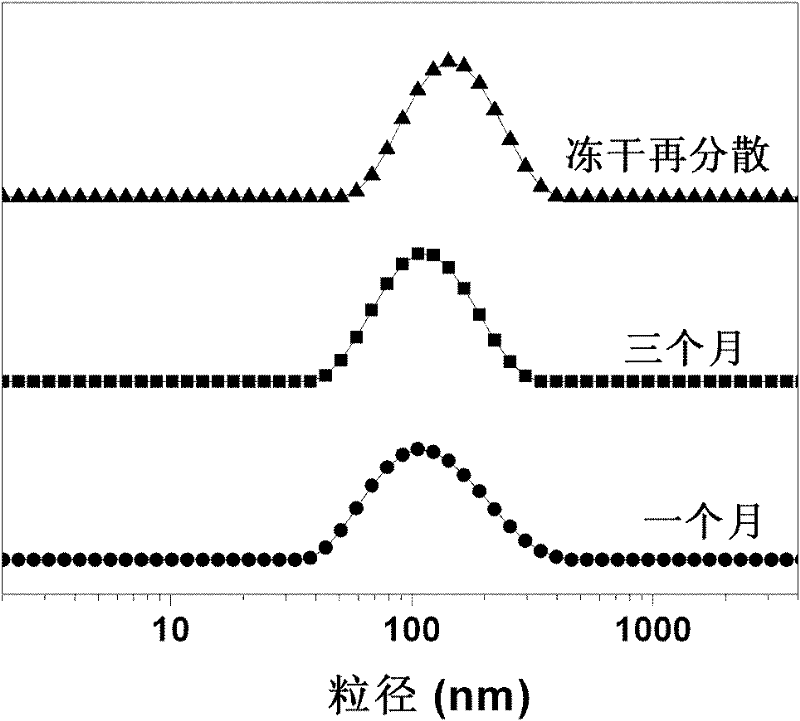
Lyophobic and modified glucan-modified long circulating liposome and preparation method thereof
A long-circulating liposome and hydrophobic modification technology, which is applied in the directions of liposome delivery, medical preparations of inactive ingredients, and pharmaceutical formulations, can solve the problems of high cost and difficult functional modification, and achieves low cost, The effect of improving active targeting function and enhancing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Hydrophobically modified dextran modified liposomes
[0045] Accurately weigh 40 mg of soybean lecithin or egg yolk lecithin and 15 mg of cholesterol, and dissolve them in 2 mL of chloroform solvent. Weigh 6 mg of hydrophobically modified dextran and dissolve it in 1 mL of methanol solvent, mix the two in a 50 mL round-bottomed flask, and remove the organic solvent by rotary evaporation until the dry lipid film is evenly deposited on the bottle wall. Hydrate with deionized water (or PBS, ammonium sulfate solution), and sonicate at 37° C. for 2 hours to obtain a bluish opalescent suspension. Then filter twice with 0.45 μm and 0.2 μm filter membranes respectively to obtain a stable hydrophobically modified dextran-modified liposome suspension.
[0046] The average particle diameter of the liposome liquid is 105 nm, and the polydispersity coefficient is 0.15.
Embodiment 2
[0048] Hydrophobic Modified Dextran Modified Liposomes Loading Ibuprofen and Other Substances
[0049] First, accurately weigh 40 mg of soybean lecithin or egg yolk lecithin, 15 mg of cholesterol, and dissolve them in 2 mL of chloroform; weigh 6 mg of hydrophobically modified dextran and dissolve them in 0.5 mL of methanol; weigh a certain amount of ibuprofen solution In 0.5mL of methanol, the three were mixed in a 50mL round bottom flask to remove the organic solvent by rotary evaporation until the dry lipid film was evenly deposited on the bottle wall. Then hydrated, and ultrasonicated at 37°C for 2 hours to obtain a liposome suspension loaded with ibuprofen.
[0050] The method for encapsulating ibuprofen as above was used to encapsulate other various substances, and the specific results are shown in Table 1.
[0051] Table 1 The loading of hydrophobically modified dextran liposomes on various substances such as ibuprofen
[0052]
Embodiment 3
[0054] Preparation of hydrophobically modified dextran liposomes loaded with doxorubicin by ammonium sulfate gradient method
[0055] The modified dextran liposome loaded with doxorubicin first makes the liposome modified by hydrophobically modified dextran with example 1, wherein 250mM (NH 4 ) 2 SO 4 Solution, get the liposome liquid at 45 ℃, dialyze in PBS (pH7.4, 50mM) for 24 hours, remove the unencapsulated (NH 4 ) 2 SO 4 . Take out the dialysate in a volumetric flask, weigh different amounts of doxorubicin, dissolve it in deionized water, add it to the volumetric flask, and dilute to the required amount with PBS, and incubate at 45°C for 1 hour to obtain hydrophobically modified dextran. Adriamycin liposomal fluid.
[0056] The average particle size of the doxorubicin-loaded modified dextran-modified liposome is about 100-150 nm, the encapsulation efficiency is as high as over 95%, and the maximum drug-loading capacity can reach 56%.
[0057] The above method was u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com