Broad spectrum type influenza vaccine and preparation method thereof
A technology of influenza A and vaccine, which is applied in the field of bioengineering, can solve the problem that the virus has no preventive and protective effect, and achieve the effect of high safety, low toxicity and side effects, and good tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
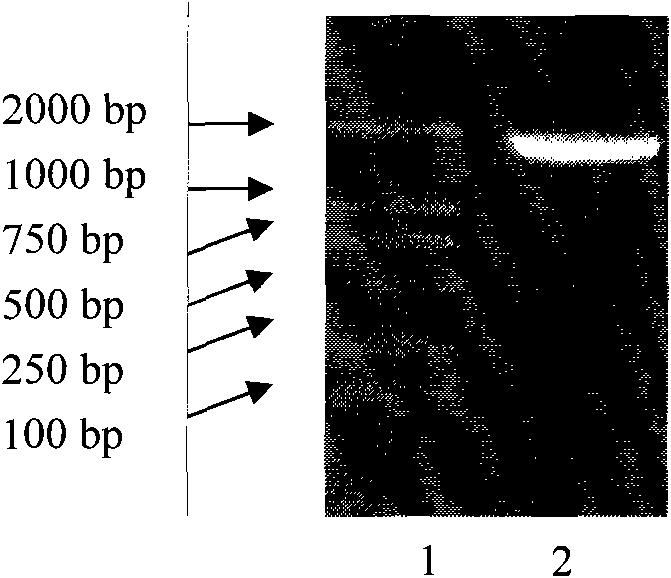
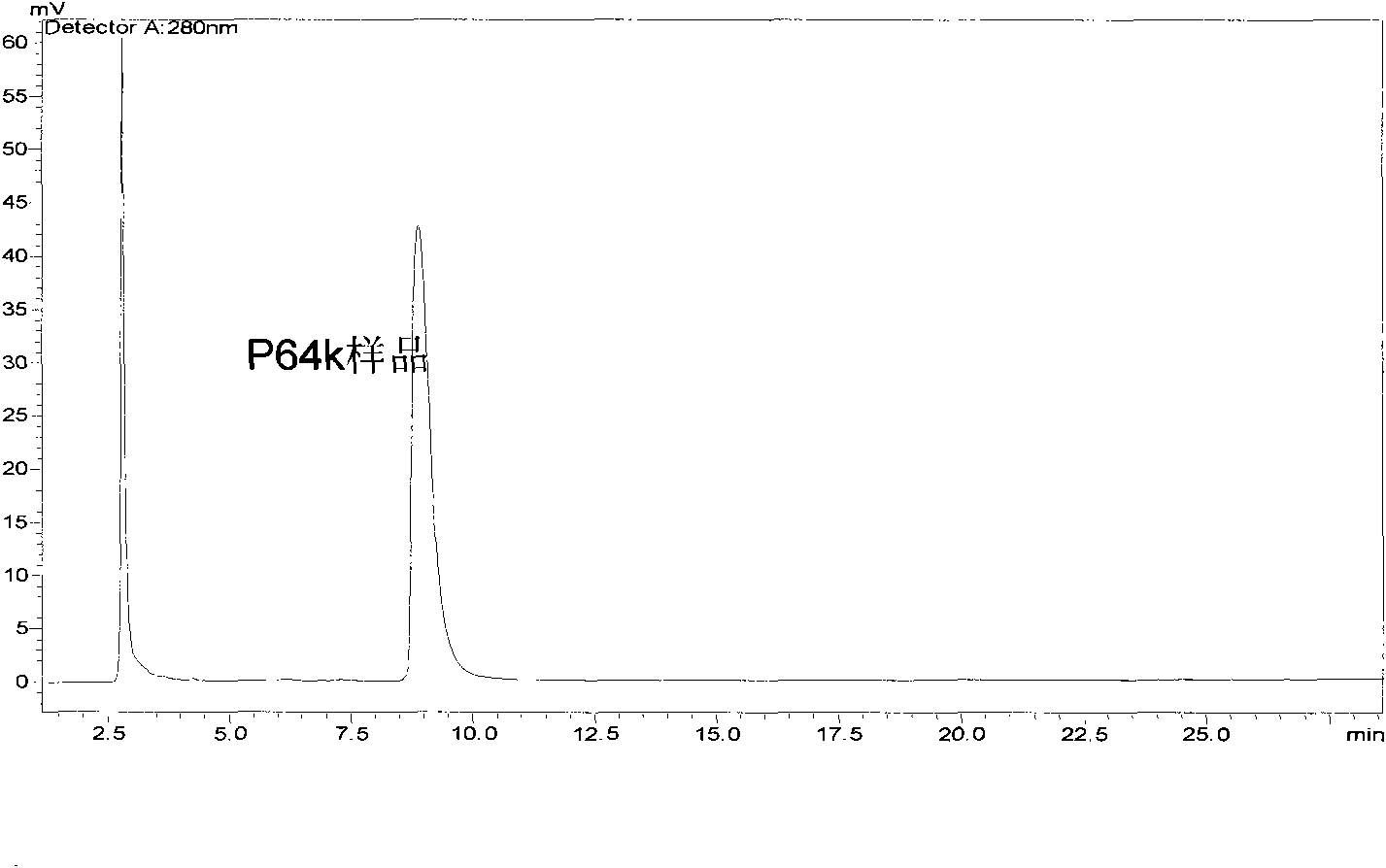
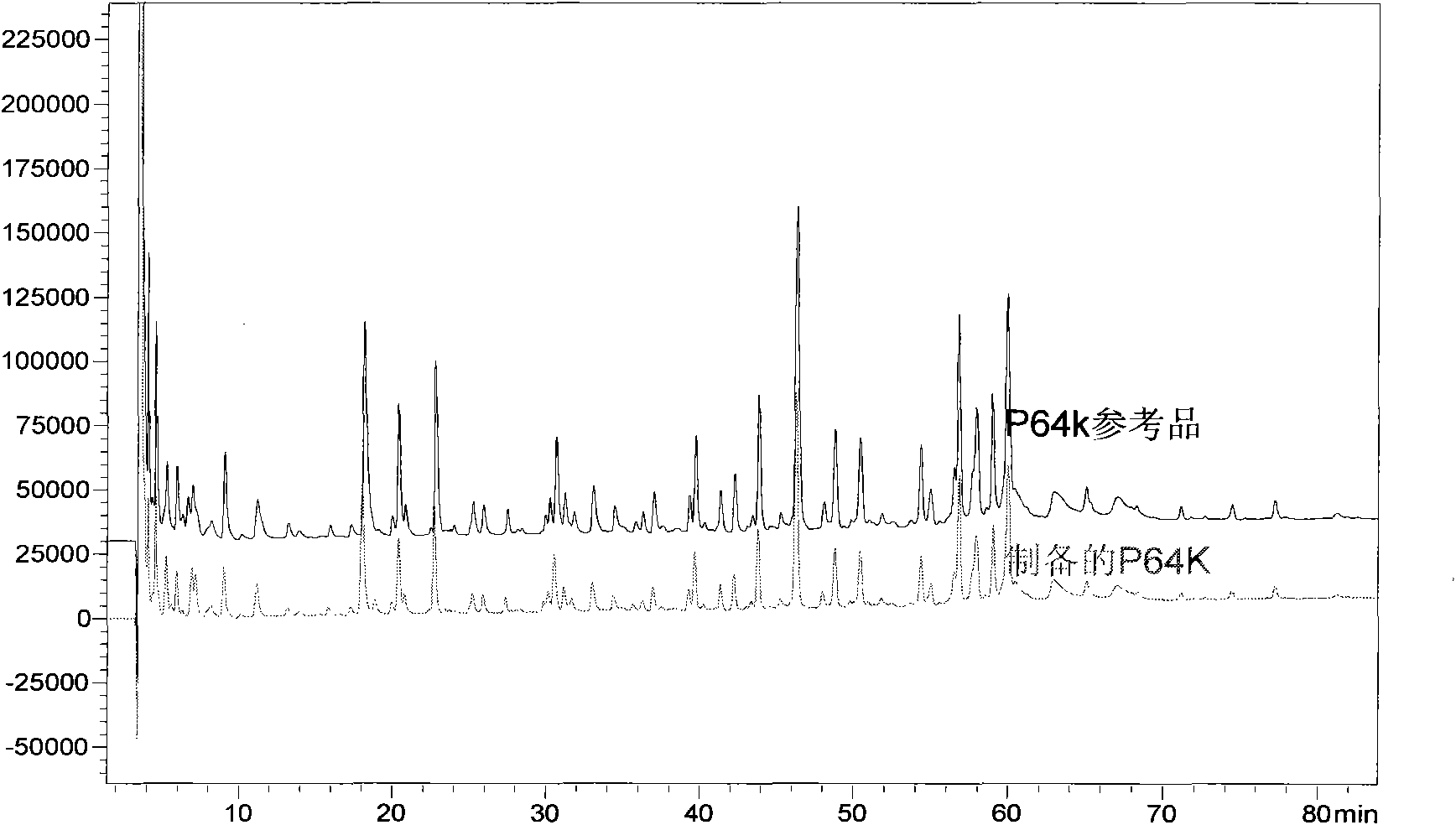
[0082] Embodiment 1, the preparation of Neisseria meningitidis outer membrane protein (P64K)
[0083] 1. Cloning of the gene encoding the outer membrane protein (P64K) of Neisseria meningitidis
[0084] According to the sequence of Neisseria meningitidis of GenBank Accession No.X77920.1, primers were designed using Primer5.0 software, upstream primer: 5-CATG CCATGG CTTTAGTTGAATTGAA-3, downstream primer: 5-CCG GAATTC TTATTTTTTCTTTTGCGGAG-3, where the underlined parts are the restriction sites of NcoI and EcoRI respectively. Neisseria meningitidis CMCC 29336 (purchased from China Medical Bacteria Collection Center, referred to as CMCC) was boiled at 100°C for 10 min, and 3 μl was taken as a template for PCR amplification. The PCR reaction system is: 3 μl template, 5 μl 10×PCR buffer, 1 μl 10 mmol / L dNTP, 1 μl Pyrobest high-fidelity DNA polymerase (Shanghai Sangong product), 0.5 μl each of upstream and downstream primers with a final concentration of 0.5 μmol / L, adding Ultra...
Embodiment 2
[0091] Embodiment 2, the preparation of M2e peptide
[0092] 1. Solid phase synthesis of M2e peptide:
[0093] Taking the amino acid Cys at the C-terminus of the M2e peptide sequence SLLTEVETPTRSEWECRCSDSSDC[SEQ ID NO: 1] as the initial reactant, the NH 2 Protect with Fmoc, react with carrier Trityl chloride resin (also Rink Amide resin or Wang resin), the molar ratio is 1:1.5, use DCM or DMF as the reaction solvent, mix under alkaline conditions, and react for about 2 hours; The neutral solvent piperidine removes the protective group Fmoc connected to the first amino acid amino group on the carrier, rinses with DMF to remove excess amino acids, Fmoc and other small molecules; then add the activator DIC / HOBT mixture to activate Cys N-terminal After the free amino group is activated for 15 minutes, the second amino acid (see SEQ ID NO: 1) is added and cycled sequentially to complete the connection of all amino acids on the M2e peptide. After the synthesis is completed, triflu...
Embodiment 3
[0096] Example 3, Preparation of M2e peptide-P64K conjugate
[0097] 1. Activation of P64K:
[0098] Using SMCC (Succinimidyl-4-(N-maleimidomethyl)cydohexane-1-carboxylate) as an activator, mix P64K:SMCC with a molar ratio of 1:20 in phosphate buffer at pH 7.2, stir at room temperature for 2 hours, add An appropriate amount of glycine was used to terminate the reaction, and then the activated P64K was ultrafiltered with an ultrafiltration cup (MW: 30KDa) to remove unreacted SMCC and excess glycine.
[0099] 2. Preparation and purification of conjugates
[0100] Activated P64K was reacted with M2e at a molar ratio of 1:15 at a known concentration at pH 6.5 at 4° C. for 24 h, and the reaction was terminated with mercaptoethanol. Purify the obtained conjugate with an ultrafiltration cup (MW: 30KDa) to remove unreacted M2e and mercaptoethanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com