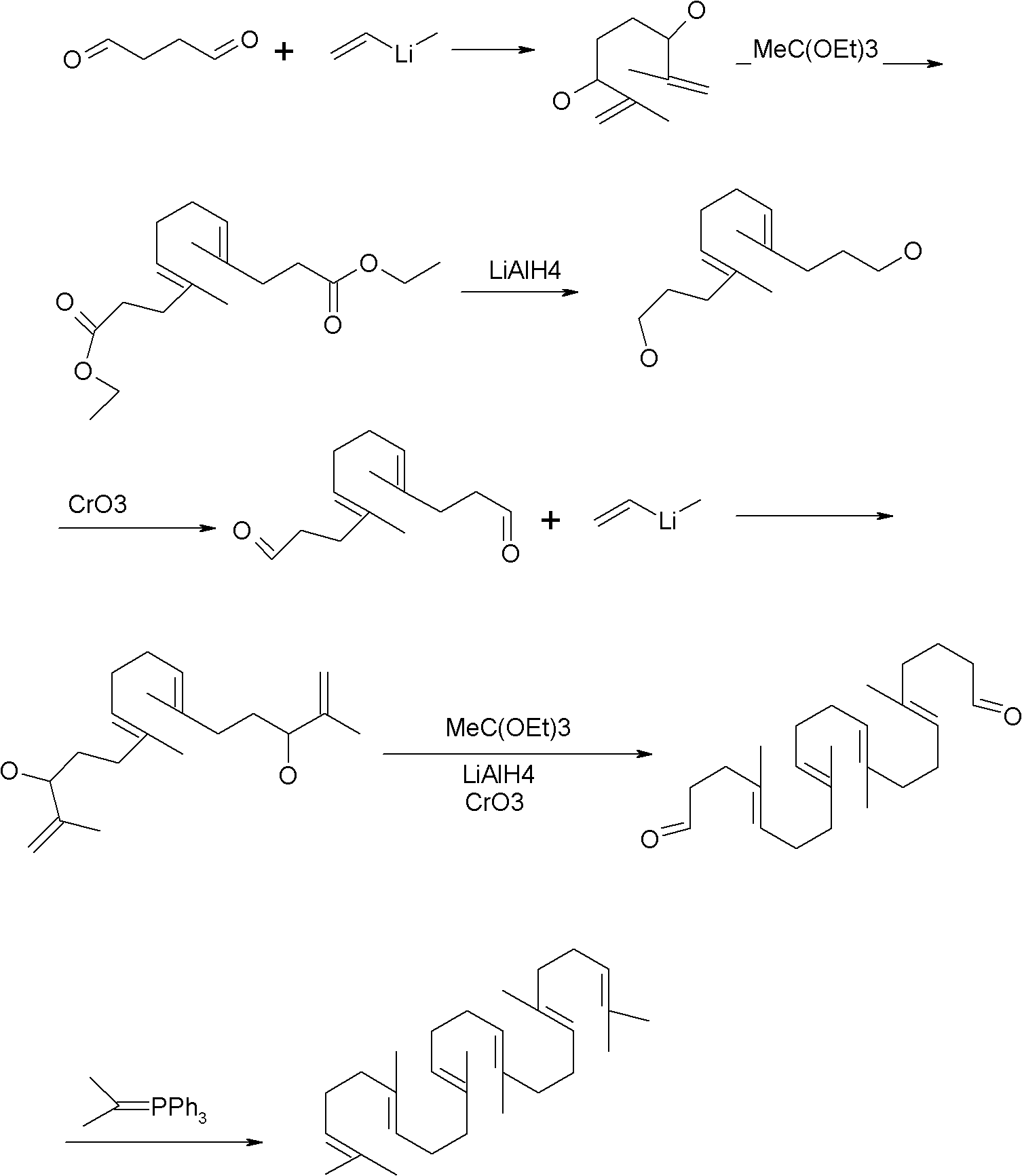
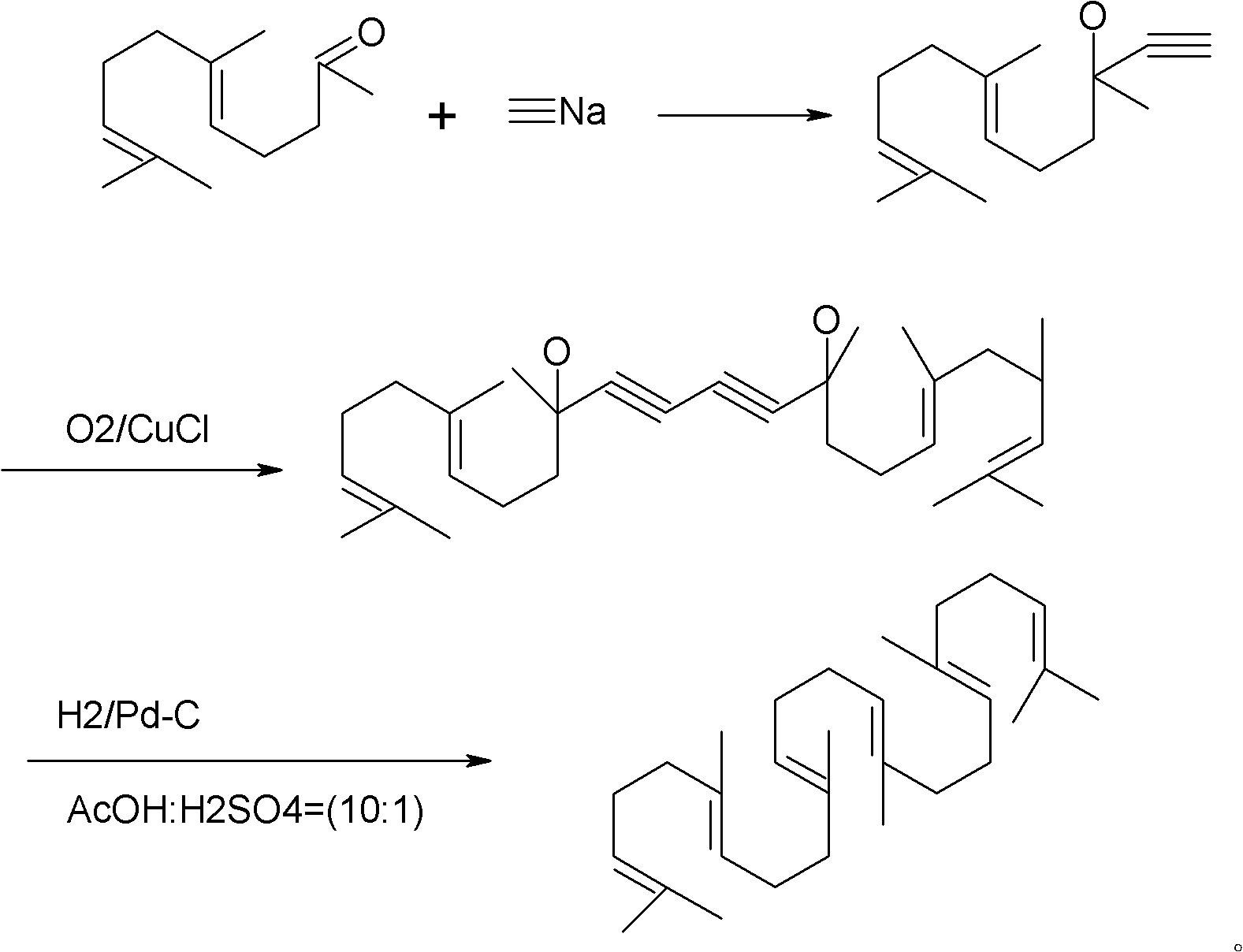
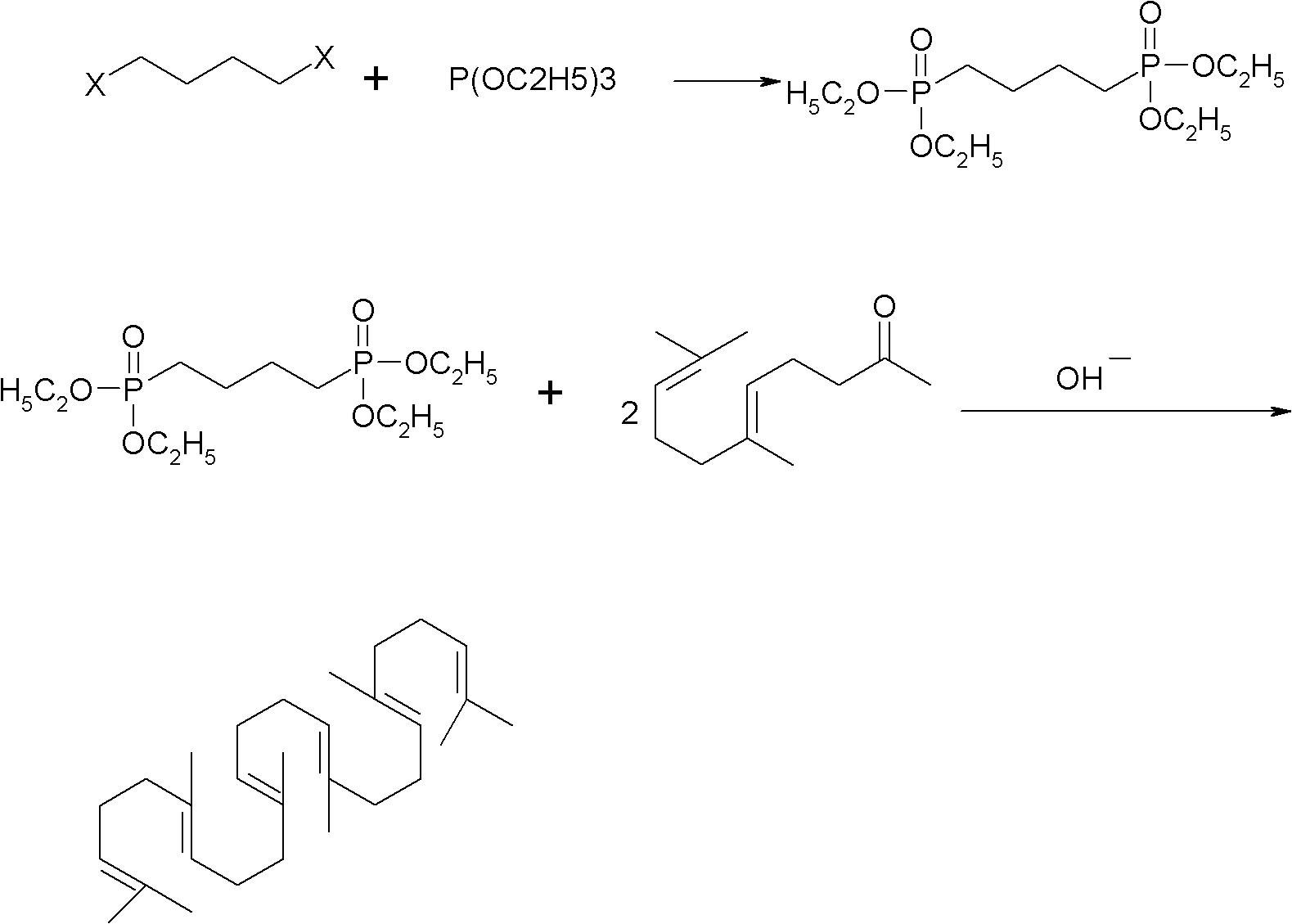
Method for synthesizing squalene
A synthesis method and technology of squalene, applied in chemical instruments and methods, hydrocarbons, hydrocarbons, etc., can solve the problems of explosion of polymerization reaction, unfavorable industrial production, harsh reaction requirements of sodium acetylene, etc., and improve safety. , The advantages of industrialization are obvious and the operation is convenient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Put 41.5g (0.25mol) of triethyl phosphite into a four-necked flask, heat it in an oil bath to 110°C, add 12.7g (0.1mol) of dichlorobutane dropwise, after the drop, raise the temperature to 180°C, and keep warm 6.0 hours of reaction. Sampling, gas phase detection to stop the reaction when the intermediate is less than 0.2%. A distillation unit was connected to remove the low boilers to obtain 30 g of crude oil with a yield of 90.9%.
[0024] 2) Dissolve 30g of crude oil in 200ml of toluene and cyclohexane mixed solvent (1:1), add 48.5g (0.25mol) geranyl acetone, stir, cool to 30°C, slowly add 10.8g (0.2mol) ) sodium methoxide, keep the temperature at about 50° C., keep it warm for 5.0 hours, drop ice water into the reaction container, stir for half an hour, and let stand to separate layers. The water layer was separated, and the organic layer was washed with water, washed with brine, dried over anhydrous magnesium sulfate, and concentrated to obtain 35 g of crude pr...
Embodiment 2
[0026] 1) Put 41.5g (0.25mol) of triethyl phosphite into a four-necked flask, heat it in an oil bath to 140°C, add 12.7g (0.1mol) of dichlorobutane dropwise, after the drop, raise the temperature to 180°C, and keep warm 6.0 hours of reaction. Sampling, gas phase detection to stop the reaction when the intermediate is less than 0.2%. Connect the distillation unit to remove low boilers to obtain 31 g of crude oil with a yield of 93.9%.
[0027] 2) Dissolve 31g of crude oil in 200ml of toluene and cyclohexane mixed solvent (1:2), add 48.5g (0.25mol) geranyl acetone, stir, cool to 30°C, slowly add 12g (0.3mol) Sodium hydroxide, keep the temperature at about 50°C, keep warm for 5.0 hours, drop ice water into the reaction container, stir for half an hour, let stand and separate into layers. The water layer was separated, and the organic layer was washed with water, washed with brine, dried over anhydrous magnesium sulfate, and concentrated to obtain 36 g of crude product, which wa...
Embodiment 3
[0029] 1) Put 41.5g (0.25mol) of triethyl phosphite into a four-necked flask, heat it in an oil bath to 140°C, add 12.7g (0.1mol) of dichlorobutane dropwise, after the drop, raise the temperature to 180°C, and keep warm 6.0 hours of reaction. Sampling, gas phase detection to stop the reaction when the intermediate is less than 0.2%. A distillation unit was connected to remove the low boilers to obtain 30 g of crude oil with a yield of 90.9%.
[0030] 2) Dissolve 30g of crude oil in a mixed solvent (1:1) of 200ml toluene and cyclohexane, add 48.5g (0.25mol) geranyl acetone, stir, cool to 30°C, slowly add 41.4g (0.3mol) ) Potassium carbonate, keep the temperature at about 50° C., keep the temperature for 8.0 hours to react, drop ice water into the reaction container, stir for half an hour, and let stand to separate layers. The water layer was separated, and the organic layer was washed with water, washed with brine, dried over anhydrous magnesium sulfate, and concentrated to o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com