Low-temperature alpha-galactosidase GalA17, gene thereof and application thereof
A galactosidase and low-temperature technology, applied in the field of genetic engineering, can solve the problem of inability to hydrolyze various substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Screening of Flavobacterium sp.TN17
[0047] The primary screening of cellulase and hemicellulase production activities of commensal microorganisms in the gastrointestinal tract of longicorn beetles was carried out by Congo red method. The more enzymes produced or the higher the enzyme activity, the larger the hydrolysis circle; the faster the enzyme production, the earlier the hydrolysis circle appears. A total of 100 strains of bacteria were randomly selected on the plate. The genomes of the 100 bacterial strains were extracted and amplified to obtain 16S rDNA. After comparison by BLASTn, the strain TN17 belonged to the genus Flavobacterium and was named Flavobacterium sp.TN17.
Embodiment 2
[0048] Example 2 Clone of Flavobacterium sp.TN17α-galactosidase coding gene galA17
[0049] Extraction of Flavobacterium sp.TN17 genomic DNA:
[0050] Filter the cultured bacteria for 1 day with sterile filter paper and put them into a mortar, add 2mL extract, grind for 5min, then put the grinding solution in a 50mL centrifuge tube, lyse in a water bath at 65°C for 20min, and mix well every 10min , centrifuged at 10000 rpm for 5 min at 4°C. The supernatant was extracted in phenol / chloroform to remove impurity proteins, and then an equal volume of isopropanol was added to the supernatant. After standing at room temperature for 5 minutes, centrifuge at 10,000 rpm for 10 minutes at 4°C. The supernatant was discarded, the precipitate was washed twice with 70% ethanol, dried in vacuum, dissolved by adding an appropriate amount of TE, and stored at -20°C for later use.
[0051] According to the conservation of α-galactosidase genes in family 36 ([F / L / V]-[L / V]-[L / M / V]-D-D-G-W-F and...
Embodiment 3
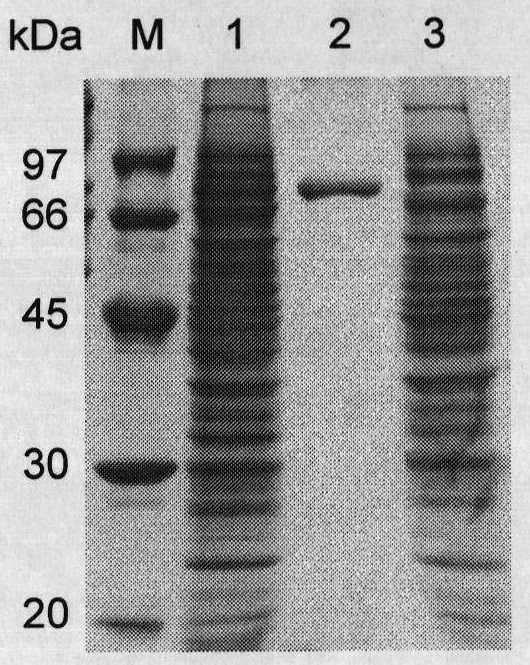
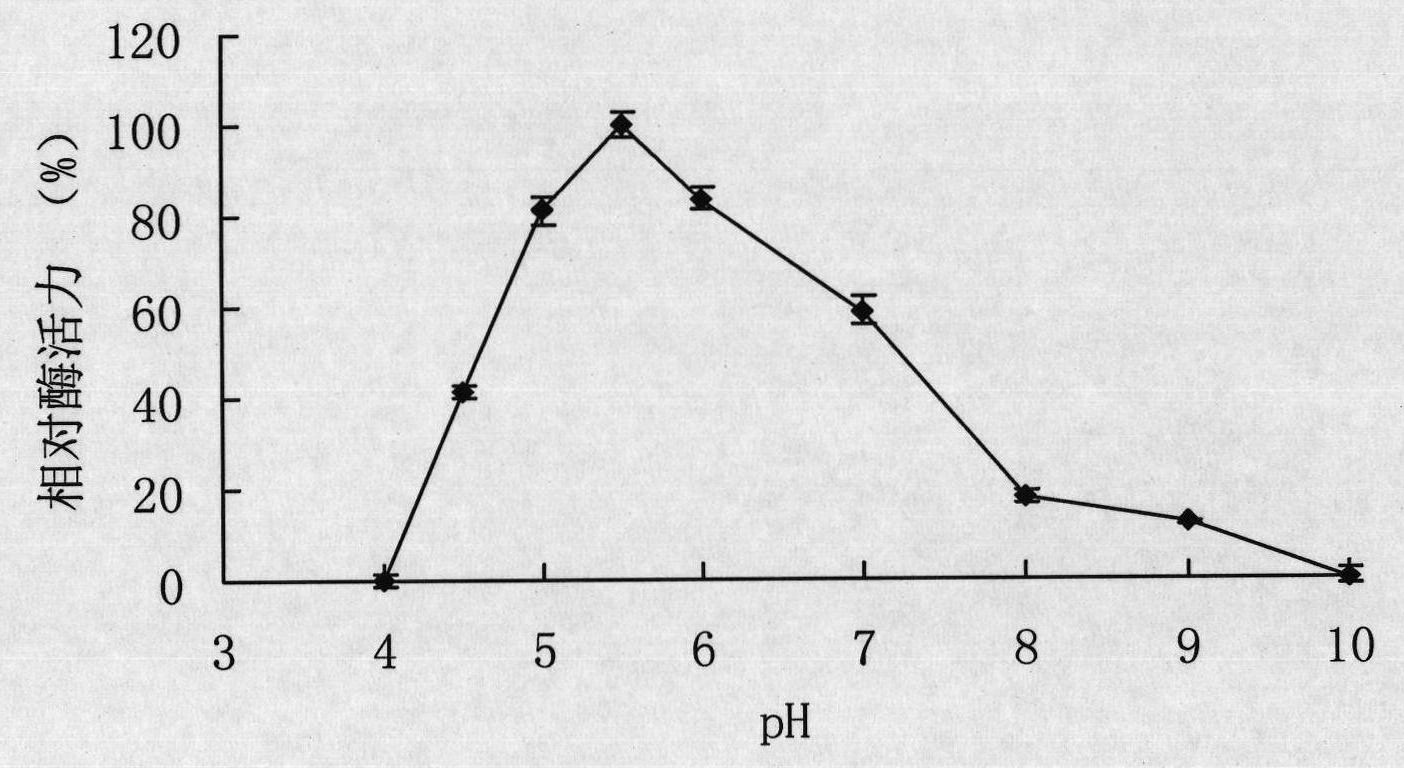
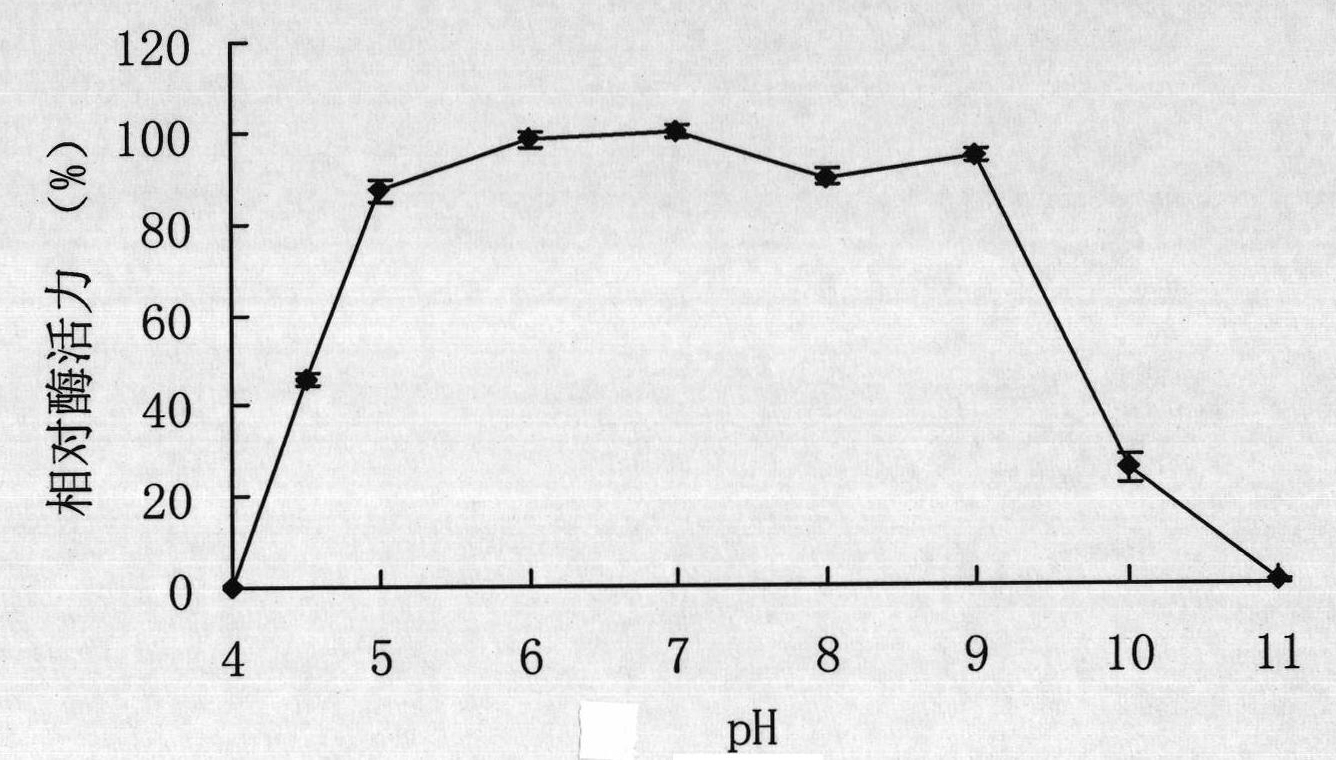
[0059] Example 3 Preparation of recombinant α-galactosidase.
[0060] The expression vector pET22b(+) was double digested (BamHI+HindIII), and the gene galA17 encoding α-galactosidase was double digested (BamHI+HindIII) to cut out the gene encoding mature α-galactosidase The fragment was connected with the expression vector pET22b(+), and the recombinant plasmid pET-galAl7 containing the gene galA17 of Flavobacterium sp.TN17α-galactosidase was obtained and transformed into Escherichia coli BL21 (DE3), and the recombinant Escherichia coli strain BL21 / galA17 was obtained.
[0061] Take the E.coli BL21(DE3) strain containing the recombinant plasmid pET-galA17 and the E.coli BL21(DE3) strain containing only the pET-22b(+) empty plasmid, and inoculate them in LB (containing 100 μg / mLAmp) medium, shake rapidly at 37°C for 16h. Then inoculate this activated bacterial solution into fresh LB (containing 100 μg / mL Amp) culture solution with 1% inoculum, and cultivate it with rapid sha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com