Difunctional electrocatalyst with heterostructure nickel cobalt nitride nanosheet array and preparation and application of difunctional electrocatalyst
A nanosheet array, heterostructure technology, applied in nanotechnology, electrodes, electrolysis process, etc., to achieve the effect of improving catalytic activity, promoting charge transfer, and fast charge transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of precursor nickel cobalt hydroxide, nickel hydroxide, cobalt hydroxide:
[0048] Weigh 0.476g nickel chloride hexahydrate, 0.476g cobalt chloride hexahydrate and 0.296g ammonium fluoride, dissolve 0.96g urea in 45mL deionized water to form a uniform mixed solution, add this solution to the reaction containing clean carbon cloth The nickel-cobalt bimetallic precursor supported on the carbon cloth can be obtained after hydrothermal reaction at 120°C for 5 hours, and dried in a vacuum oven at 80°C for later use. Similarly, only a single metal salt precursor (corresponding to the addition of only nickel chloride hexahydrate and cobalt chloride hexahydrate, respectively) was added in the hydrothermal process to synthesize nickel nanosheets and cobalt nanowire precursors supported on carbon cloth.
Embodiment 2
[0050] Preparation of nickel-cobalt nitride heterojunction nanosheet bifunctional catalyst and nickel-cobalt nitride catalyst:
[0051] The nickel-cobalt precursor in Example 1, the nickel precursor, and the cobalt precursor are gasified with nitrogen in an ammonia atmosphere to obtain Co-Ni 3 N / CC bifunctional electrocatalysts, and comparative samples of nickel and cobalt nitride catalysts, wherein the nitriding temperature is 500°C, the nitriding time is 2h, and the heating rate is 5°C / min.
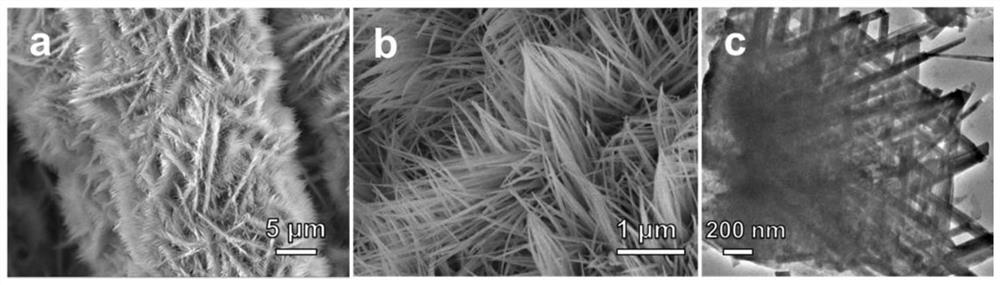
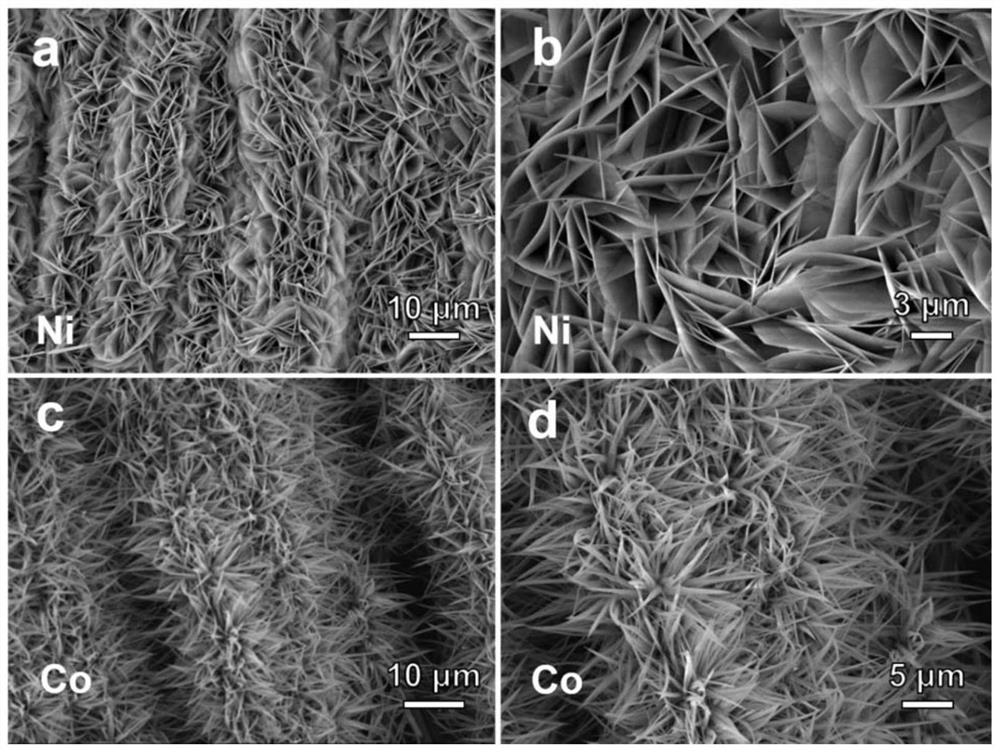
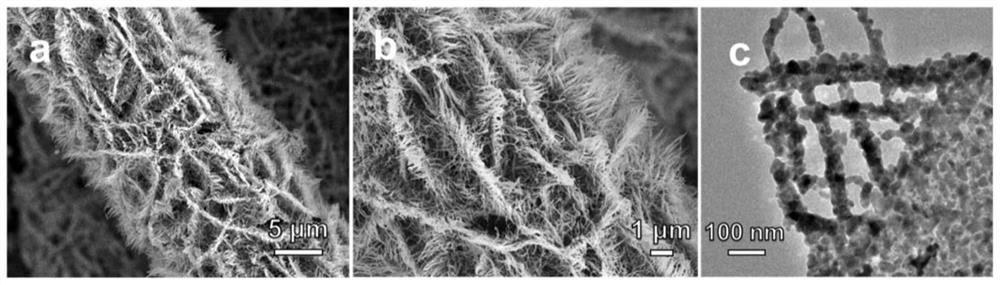
[0052] Picture 1-1 The scanning electron microscope and transmission electron microscope images of the nickel-cobalt bimetallic precursor are shown. It can be seen from the figure that the nickel-cobalt precursor is uniformly loaded on the surface of the carbon cloth, and the nickel-cobalt precursor shows a two-dimensional line sheet morphology and the surface smooth. Figure 1-2 The scanning electron microscope images of nickel and cobalt single metal precursors are shown. It can be ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com