Method for preparing flexible polyester nickel-coating electrode
A nickel electrode and polyester technology is applied in the field of preparation of flexible polyester nickel-coated electrodes, which can solve the problems of decomposition of plating solution, uneven surface of plating layer, and low surface free energy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
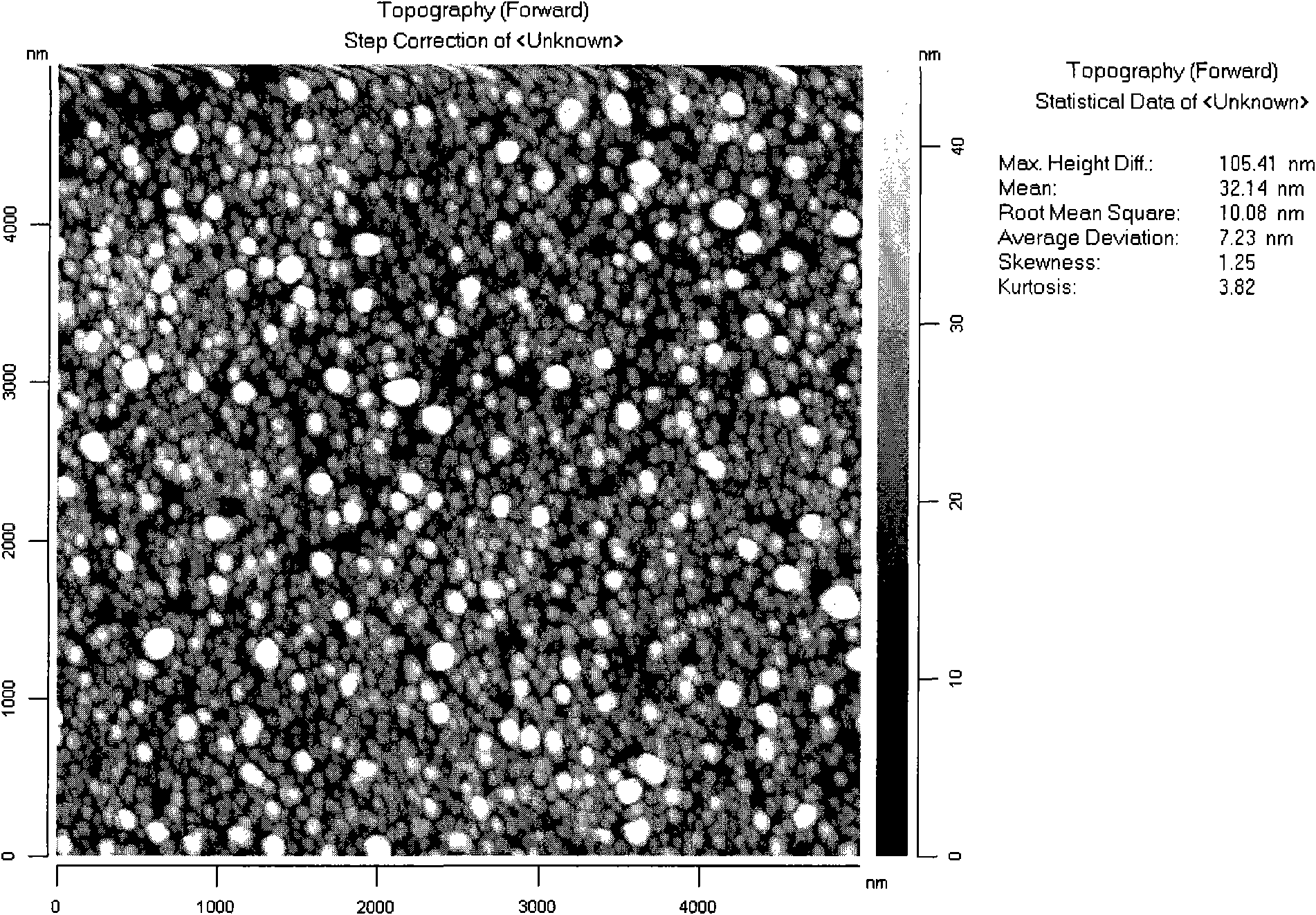
Image
Examples
Embodiment 1
[0022] Wash a polyethylene terephthalate sheet with an area of 5 cm × 10 cm with deionized water and dry it; place it in an acetone solution of 2 g / L 3-aminopropyltrimethoxysilane , soaked for 30 minutes, taken out, rinsed with acetone, and dried to obtain a polyester substrate with siliconized surface.
[0023] 3 g of silver nitrate, 6 g of glucose, and 4 mL of ammonia water (28% by weight) were dissolved in 1000 mL of deionized water to obtain a silver nitrate-glucose mixed solution. Soak the siliconized polyester substrate in the silver nitrate-glucose mixed solution for 5 seconds, take it out, wash it, and dry it.
[0024] Dissolve 20g of nickel sulfate, 18g of sodium pyrophosphate, 2g of sodium hydroxide, and 2g of N,N-dimethylaminoborane in 1000mL of deionized water to obtain a nickel electroless plating solution. Soak the silver-catalyzed polyester substrate in a nickel electroless plating solution, perform electroless plating at room temperature for 20 minutes, take...
Embodiment 2
[0026] The polytrimethylene terephthalate sheet with an area of 5 cm × 10 cm was washed with deionized water and dried; it was placed in an acetone solution of 3-aminopropyltriethoxysilane with a concentration of 3 g / L, Soak for 60 minutes, take it out, rinse with acetone, and dry to obtain a polyester substrate with siliconized surface.
[0027] 5 g of silver nitrate, 8 g of glucose, and 6 mL of ammonia water (28% by weight) were dissolved in 1000 mL of deionized water to obtain a silver nitrate-glucose mixed solution. Soak the siliconized polyester substrate in the silver nitrate-glucose mixed solution for 10 seconds, take it out, wash it, and dry it.
[0028] Dissolve 50g of nickel sulfate, 24g of sodium pyrophosphate, 4g of sodium hydroxide, and 4g of N,N-dimethylaminoborane in 1000mL of deionized water to obtain a nickel electroless plating solution. Soak the silver-catalyzed polyester substrate in a nickel electroless plating solution, perform electroless plating at r...
Embodiment 3
[0030] Wash the polybutylene terephthalate sheet with an area of 5 cm × 10 cm with deionized water and dry it; place it in an acetone solution of 1 g / L 3-mercaptopropyltrimethoxysilane , soaked for 40 minutes, taken out, rinsed with acetone, and dried to obtain a polyester substrate with siliconized surface.
[0031] 5 g of silver nitrate, 7 g of glucose, and 3 mL of ammonia water (28% by weight) were dissolved in 1000 mL of deionized water to obtain a silver nitrate-glucose mixed solution. Soak the siliconized polyester substrate in the silver nitrate-glucose mixed solution for 1 second, take it out, wash it, and dry it.
[0032] Dissolve 30g of nickel sulfate, 20g of sodium pyrophosphate, 3g of sodium hydroxide, and 3g of N,N-dimethylaminoborane in 1000mL of deionized water to obtain a nickel electroless plating solution. Soak the silver-catalyzed polyester substrate in a nickel electroless plating solution, perform electroless plating at room temperature for 40 minutes, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Peel strength | aaaaa | aaaaa |
| Root mean square roughness | aaaaa | aaaaa |
| Resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com