Preparation method for preparation containing glucosamine and application thereof
A technology of glucosamine and glucosamine hydrochloride, which is applied in the field of preparation of glucosamine-containing preparations, and can solve the problems of small side reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
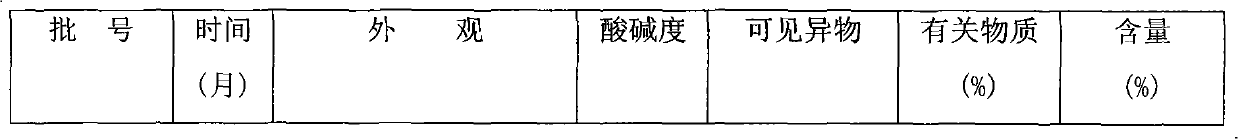
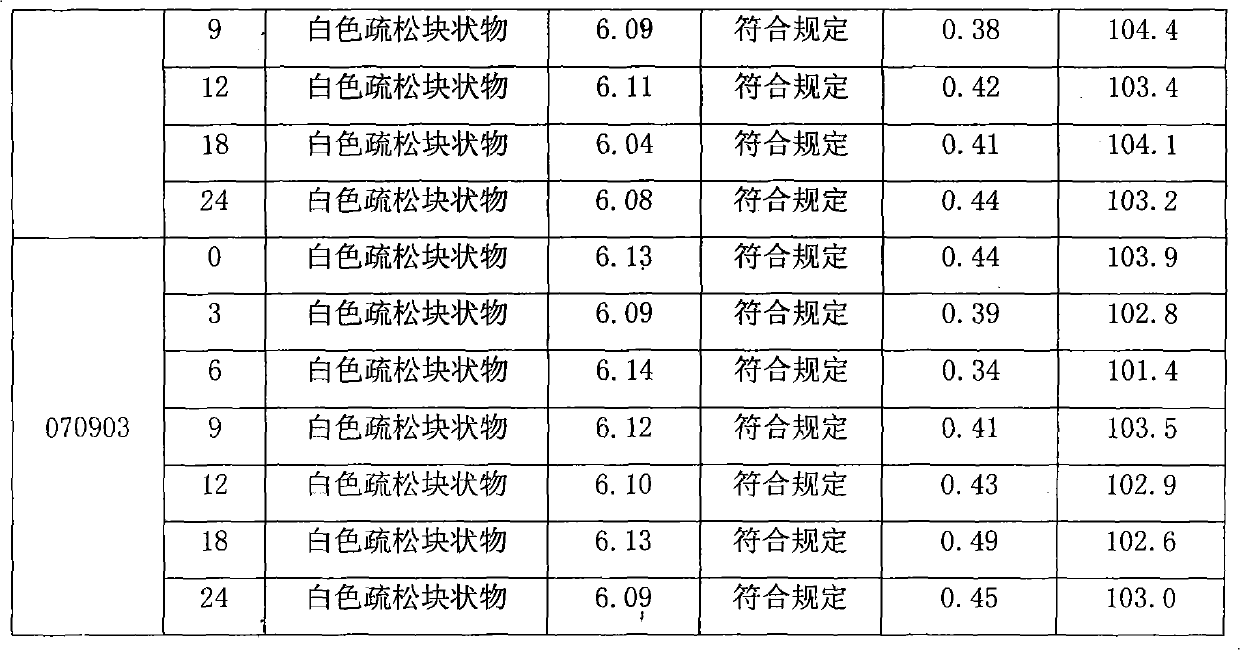
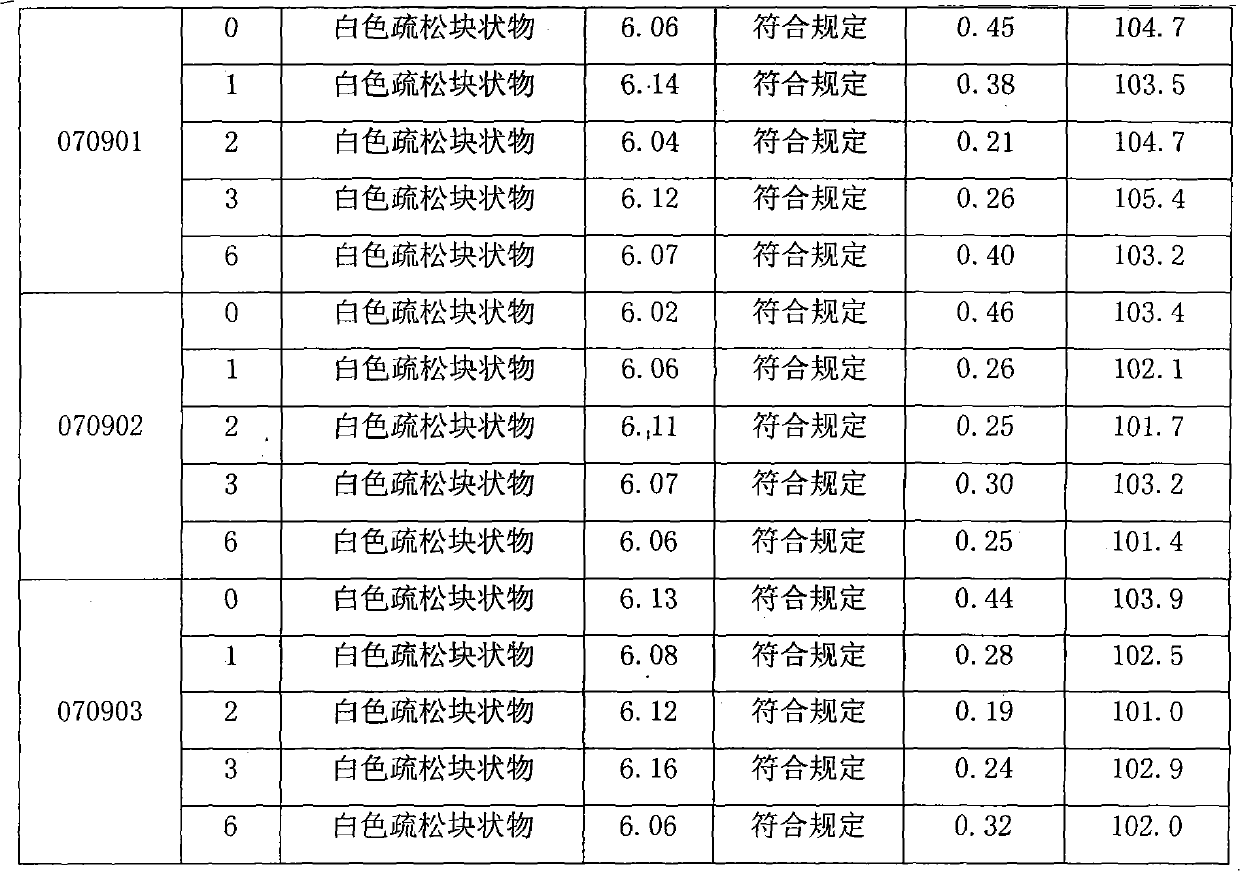
[0012] Embodiment 1: Glucosamine Hydrochloride Capsules (Specification: Glucosamine Hydrochloride 0.24g)
[0013] Glucosamine Hydrochloride 240g
[0014] Magnesium chloride 100g
[0015] Microcrystalline Cellulose 50g
[0016] 5% PVP K30 (70% ethanol) appropriate amount
[0018]
[0019] Makes 1000 capsules
[0020] Preparation process: crush each raw and auxiliary material through an 80-mesh sieve, take the prescribed amount of glucosamine hydrochloride, magnesium chloride and microcrystalline cellulose and mix evenly, add 5% PVP K30 (70% ethanol) soft material, 20 mesh granulation, 40 ~ 50 ℃ drying, 16 mesh granulation, adding magnesium stearate and mixing with the above granules, filling No. 1 capsule.
Embodiment 2
[0021] Embodiment 2: compound glucosamine sulfate chewable tablet (specification: glucosamine sulfate sodium chloride double salt 0,314g / chondroitin sulfate 0.2g)
[0022] Glucosamine Sulfate Sodium Chloride Double Salt 314g
[0023] Chondroitin sulfate (calculated as pure product) 200g
[0024] Calcium Lactate 300g
[0025] Lactose 200g
[0026] Aspartame 15g
[0027] Malic acid 10g
[0028] 2% HPMC (60% ethanol solution) appropriate amount
[0029] Orange flavor 10g
[0031]
[0032] Made into 1000 pieces
[0033] Preparation process: each raw and auxiliary material is crushed through an 80-mesh sieve, and the prescribed amount of glucosamine sulfate sodium chloride double salt, chondroitin sulfate, calcium lactate, lactose, aspartame and malic acid are mixed evenly, and 2% HPMC ( 60% ethanol solution) to make soft materials, granulate with 16 mesh, dry at 40-50°C, granulate with 12 mesh, add magne...
Embodiment 3
[0034] Embodiment 3: Glucosamine sulfate freeze-drying (glucosamine sulfate sodium chloride double salt 0.5025g)
[0035] Glucosamine Sulfate Sodium Chloride Double Salt 502.5g
[0036] Lidocaine Hydrochloride 10g
[0037] Diethanolamine 20g
[0038] Mannitol 100g
[0039] Hydrochloric acid amount
[0040] Add water for injection 3000ml
[0041]
[0042] Makes 1000 bottles
[0043] Preparation process: Take 80% water to prepare 0.04mol / L hydrochloric acid solution, add glucosamine sulfate sodium chloride double salt and stir to make it completely dissolved; add prescription amount of lidocaine hydrochloride, mannitol and diethanolamine, stir until completely dissolved, add water to a sufficient amount. Add 0.05% activated carbon, stir for 15-30 minutes, decarbonize, filter through 0.45um, 0.22um microporous membranes until clarified; fill in 3ml portions, and freeze-dry. The freeze-drying process includes: a pre-freezing process for 2-10 hours; a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com