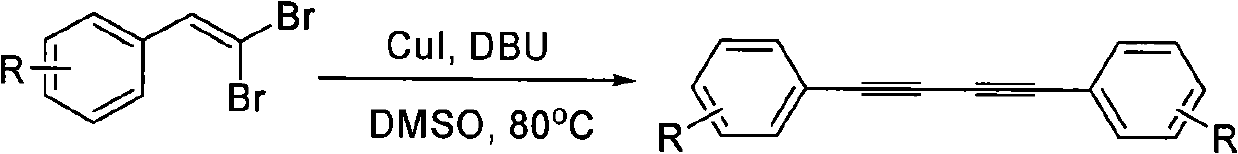
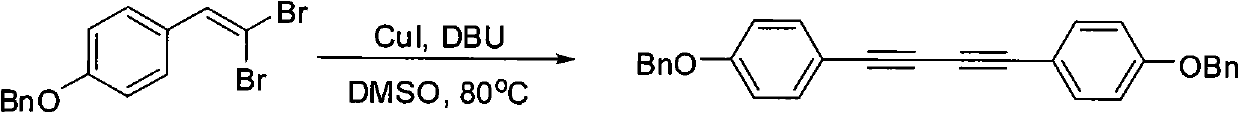Preparation method of 1,4-diaryl-1,3-butadiyne
A diaryl and diacetylene technology, applied in the preparation of amino compounds, organic compounds, organic chemical methods, etc., can solve the problems of high toxicity of catalysts and ligands, and achieve low cost, easy industrialization, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the synthesis of 1,4-bis(4-methoxyphenyl)-1,3-butadiyne
[0028]
[0029] Add 3 mL of dimethyl sulfoxide, 2-(4-methoxyphenyl)-1,1-dibromoethylene (146 mg, 0.5 mmol), DBU (115 mg, 0.75 mmol), cuprous iodide to a 60 mL sealed tube (4.75mg, 0.025mmol), stirred and reacted at 80°C for 8 hours. After the reaction, add 3mL of distilled water to the reaction solution, extract with ethyl acetate, wash the organic layer with saturated brine, dry over anhydrous sodium sulfate, remove the solvent by distillation under reduced pressure to obtain the crude product, and the crude product is subjected to column chromatography (acetic acid Ethyl ester / petroleum ether=1 / 30~1 / 5) separation to obtain 38 mg of light yellow solid 1,4-bis(4-methoxyphenyl)-1,3-butadiyne with a yield of 57%. The characterization data are as follows: 1 H NMR (500MHz, CDCl 3 ): δ=3.82(6H, s), 6.85(4H, d, J=9.0Hz), 7.46(4H, d, J=9.0Hz).
Embodiment 2
[0030] Example 2: Synthesis of 1,4-bis(4-methoxyphenyl)-1,3-butadiyne
[0031]
[0032] Add 3 mL of dimethyl sulfoxide, 2-(4-methoxyphenyl)-1,1-dibromoethylene (146 mg, 0.5 mmol), DBU (230 mg, 1.5 mmol), cuprous iodide to a 60 mL sealed tube (19mg, 0.1mmol), stirred and reacted at 80°C for 8 hours. After the reaction, add 3mL of distilled water to the reaction solution, extract with ethyl acetate, wash the organic layer with saturated brine, dry over anhydrous sodium sulfate, and remove the solvent by distillation under reduced pressure to obtain the crude product. The crude product is subjected to column chromatography (acetic acid Ethyl ester / petroleum ether=1 / 30~1 / 5) separation to obtain 59 mg of light yellow solid 1,4-bis(4-methoxyphenyl)-1,3-butadiyne with a yield of 89%. The characterization data are as follows: 1 H NMR (500MHz, CDCl 3 ): δ=3.82(6H, s), 6.85(4H, d, J=9.0Hz), 7.46(4H, d, J=9.0Hz).
Embodiment 3
[0033] Example 3: Synthesis of 1,4-bis(4-methylphenyl)-1,3-butadiyne
[0034]
[0035] Add 3 mL of dimethyl sulfoxide, 2-(4-methylphenyl)-1,1-dibromoethylene (138 mg, 0.5 mmol), DBU (230 mg, 1.5 mmol), cuprous iodide ( 19mg, 0.1mmol), stirred and reacted at 80°C for 8 hours. After the reaction, add 3mL of distilled water to the reaction solution, extract with ethyl acetate, wash the organic layer with saturated brine, dry over anhydrous sodium sulfate, and remove the solvent by distillation under reduced pressure to obtain the crude product. The crude product is subjected to column chromatography (acetic acid Ethyl ester / petroleum ether=1 / 30~1 / 5) separation to obtain 46 mg of white solid with a yield of 81%.
[0036] The characterization data are as follows: 1 H NMR (500MHz, CDCl 3 ): δ=2.35(6H, s), 7.13(4H, d, J=8.0Hz), 7.41(4H, d, J=8.0Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com