Rice dehydrin protein with antibacterial activity and application thereof
A technology of antibacterial activity, protein, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
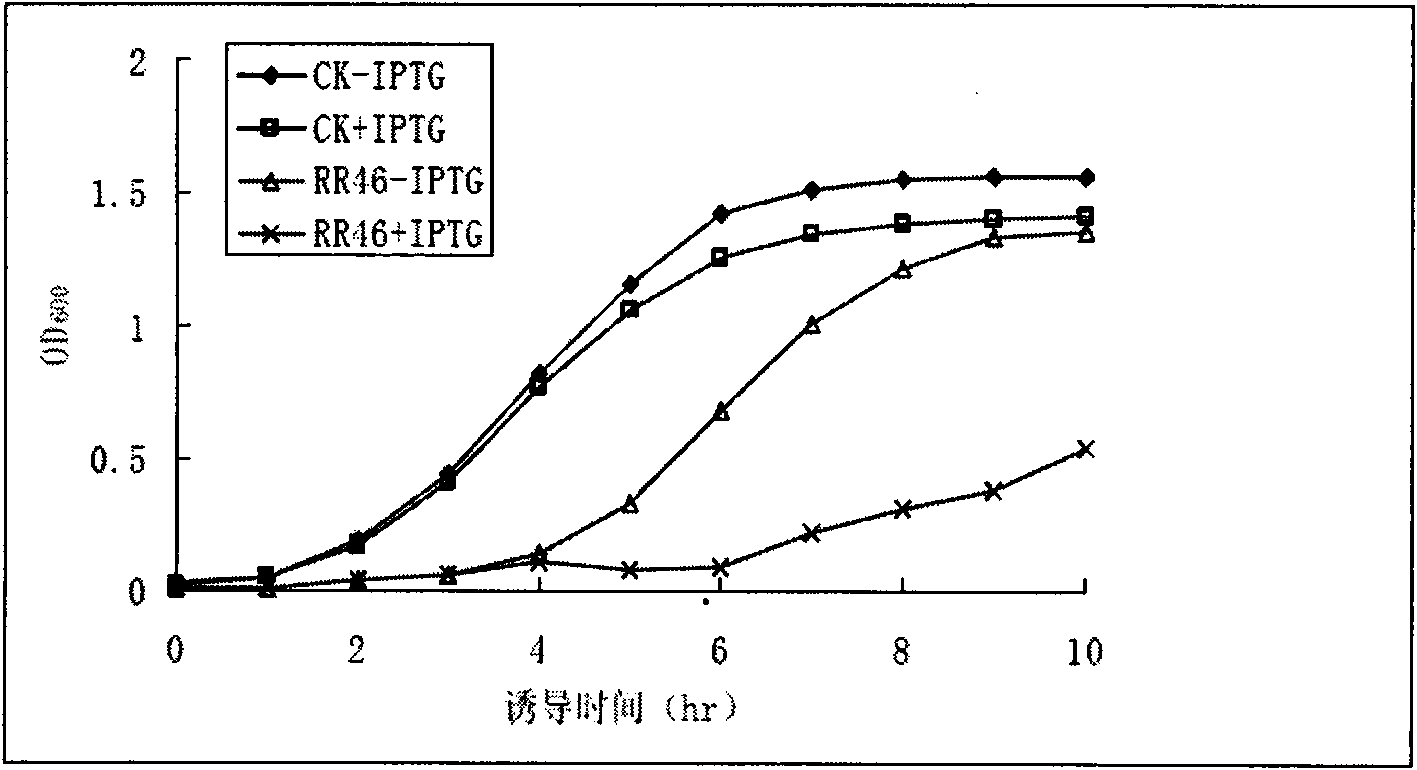
[0027] Embodiment 1, the acquisition of dehydrin clone and the growth curve of bacteria
[0028] According to the screening method of Cheng et al. (Cheng et al, 2009), the expression library from rice roots was used for screening. After induction with IPTG, the colonies that turned blue with trypan blue staining were selected. After two rounds of induced expression verification, 35 colonies were selected. The clones were terminally sequenced, and the sequencing results were analyzed by BLASTX. It was found that 7 clones encoded Dehydrin protein, 4 of which were genes encoding SK3-Dehydrin protein, and their numbers were RR46, RR51, RR142 and RR306, respectively.
[0029] Take the overnight bacteria of RR46 clone, inoculate 100 mL of LB liquid medium containing 100 μg / mL ampicillin at a ratio of 1%, shake culture at 37 ° C until the OD600 value is about 0.1, take out 50 mL and add IPTG (final concentration 1 mmol / L) to induce, and the remaining As an uninduced control, samples ...
Embodiment 2
[0030] Embodiment 2, the cloning of dehydrin gene and the expression of protein
[0031]Using the RR46 plasmid DNA as a template, the dehydrin gene was amplified and cloned into the pET28a expression vector (Zhai Congjie et al. 2009), and the cloned pET28a-Dehydrin plasmid was transformed into Escherichia coli BL21-DE3-pLysS strain, and picked Inoculate a single colony into 3 mL LB liquid medium containing 50 μg / mL kanamycin, and culture overnight at 37°C with shaking. Transfer 100 mL of LB liquid medium containing 50 μg / mL kanamycin according to 1% inoculum amount, culture at 37 °C until OD600 = 0.6-0.8, take out 50 mL and add IPTG to a final concentration of 1 mmol / L, induce for 3 hours, and culture the rest of the bacteria for the same time . Centrifuge, discard the supernatant, wash the bacteria 3 times, each time with 15mL sterile water. Resuspend in 5 mL PBS buffer, sonicate on ice (on / off=5S / 5S) for 10 min, 12000 r / min, centrifuge at 4°C for 10 min, and the supernatan...
Embodiment 3
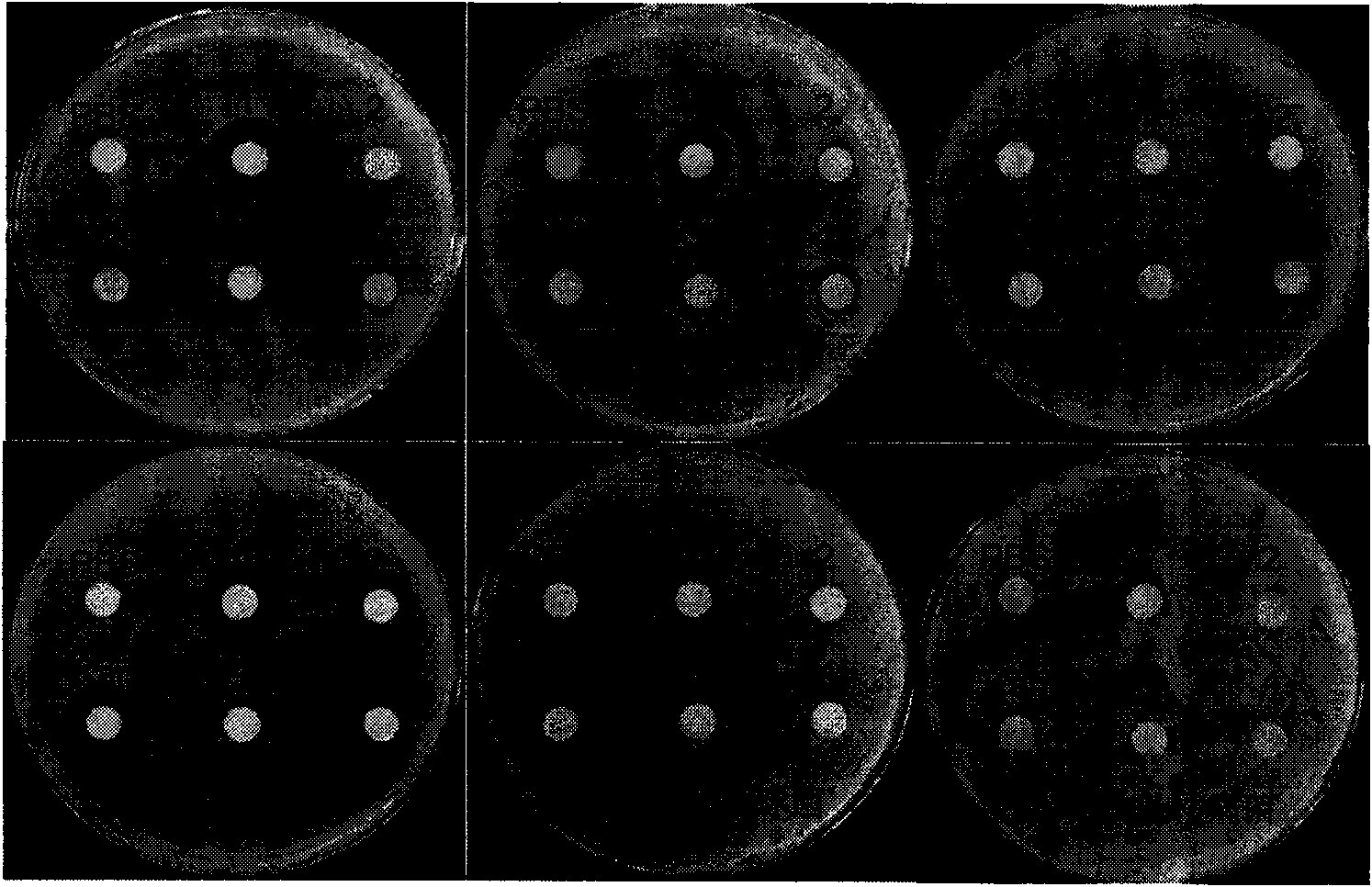
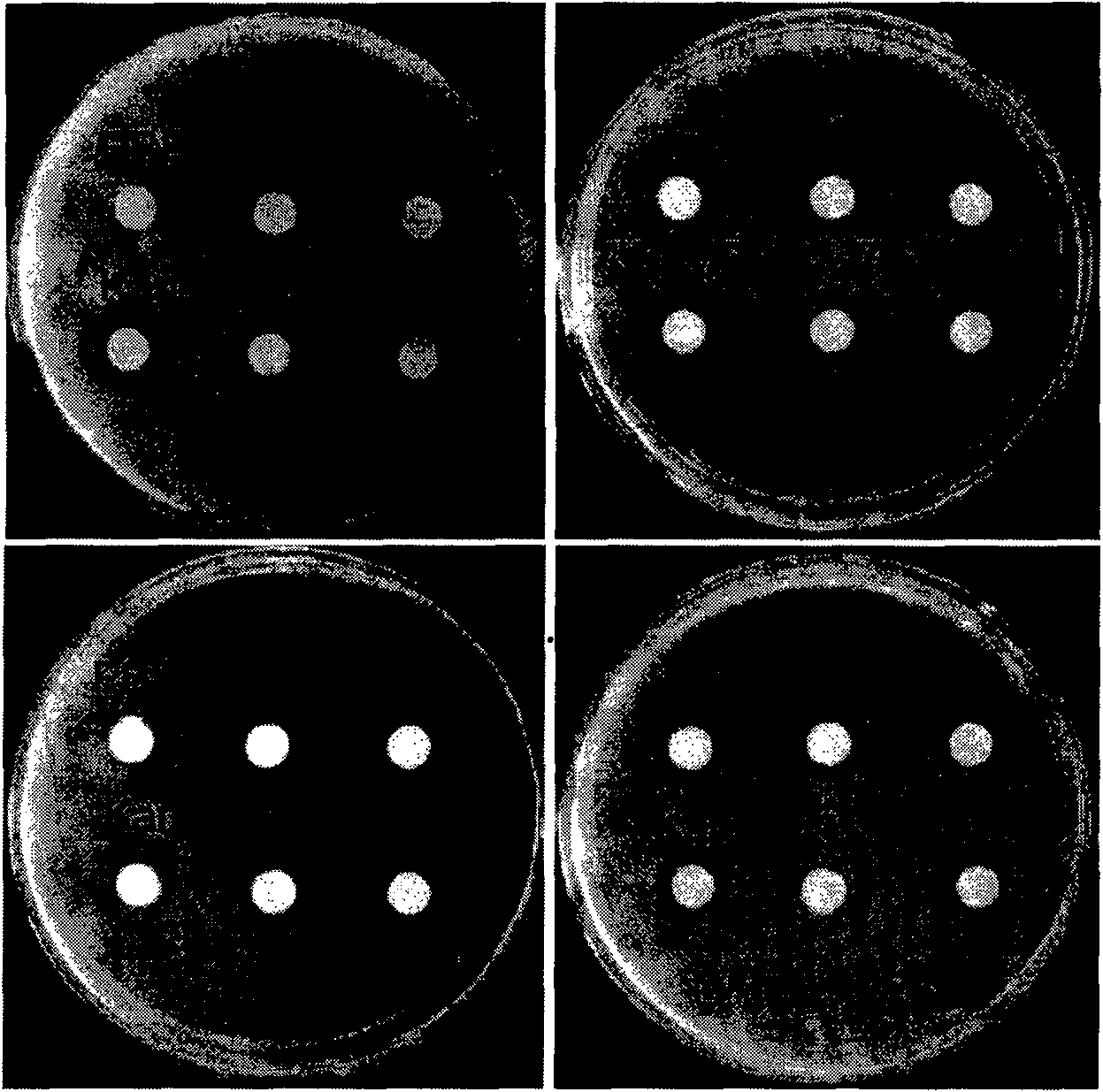
[0032] Embodiment 3, the inhibition of dehydrin protein to bacterial growth
[0033] Use a file puncher to cut a circular piece of filter paper with a diameter of about 0.7 cm, dry it after sterilization, spread it on the surface of the freshly prepared medium containing the bacteria to be tested, add 20 μL of the liquid protein to be tested on the filter paper, and keep at 37 °C Cultivate overnight, observe the inhibition zone and take pictures the next day. The results show that the bacterial lysate has obvious antibacterial effect on Bacillus pumilus, Bacillus subtilis, Sarcina luteus and Staphylococcus aureus, but it has obvious antibacterial effect on Escherichia coli, Xanthomonas oryzae had no visible antibacterial effect ( figure 2 ), the purified Dehydrin protein had no antibacterial activity (data not attached).
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com