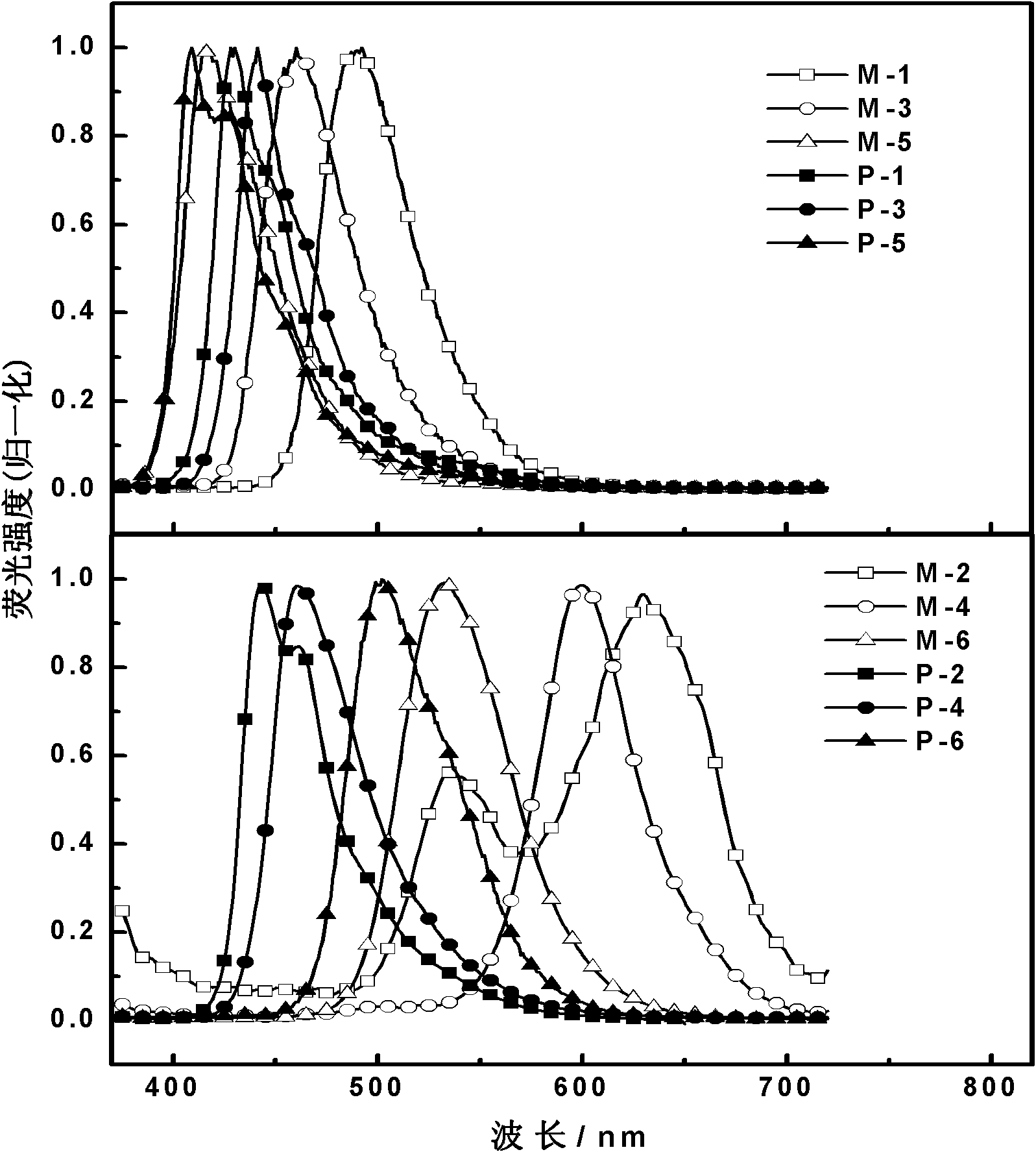
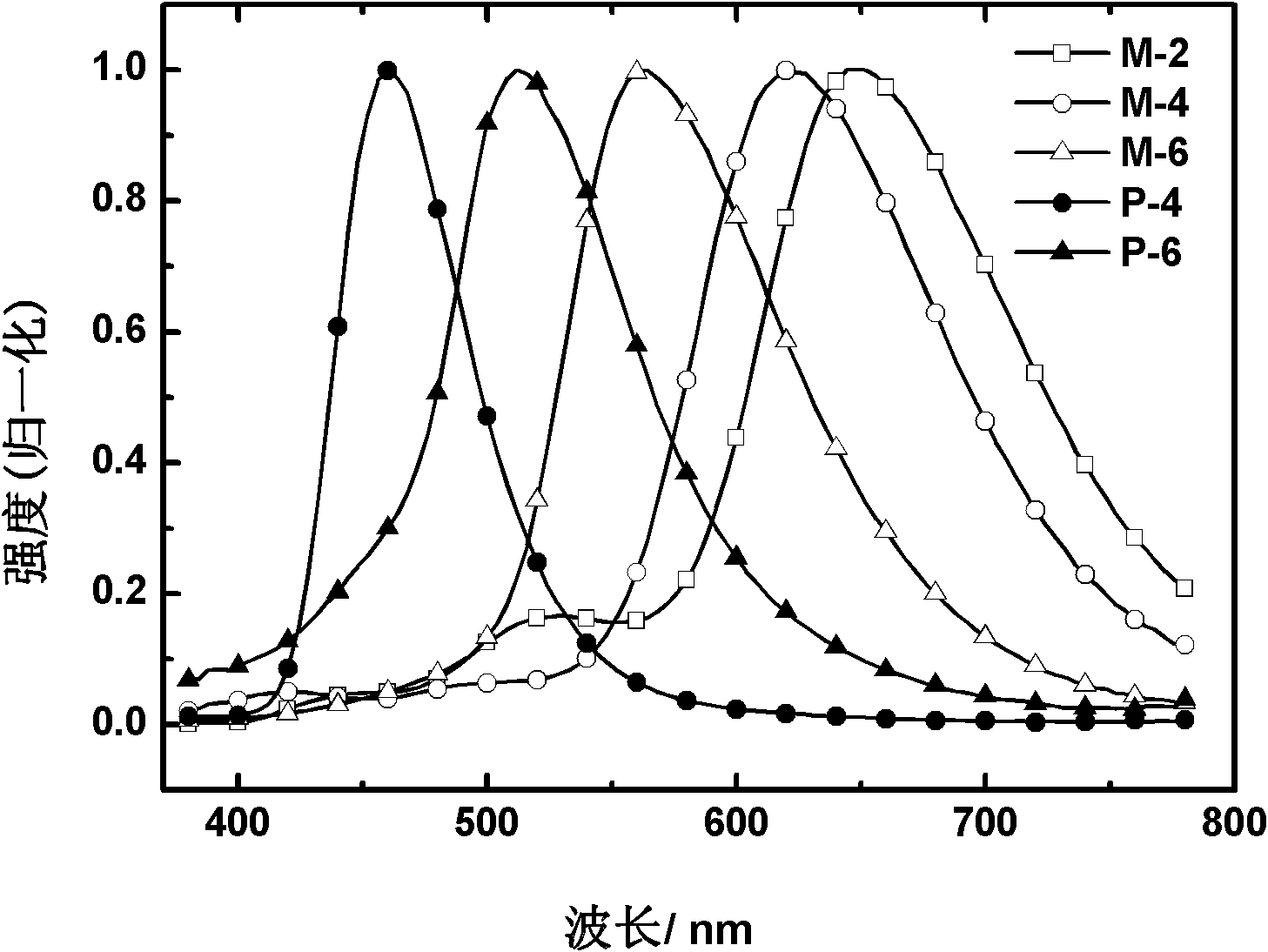
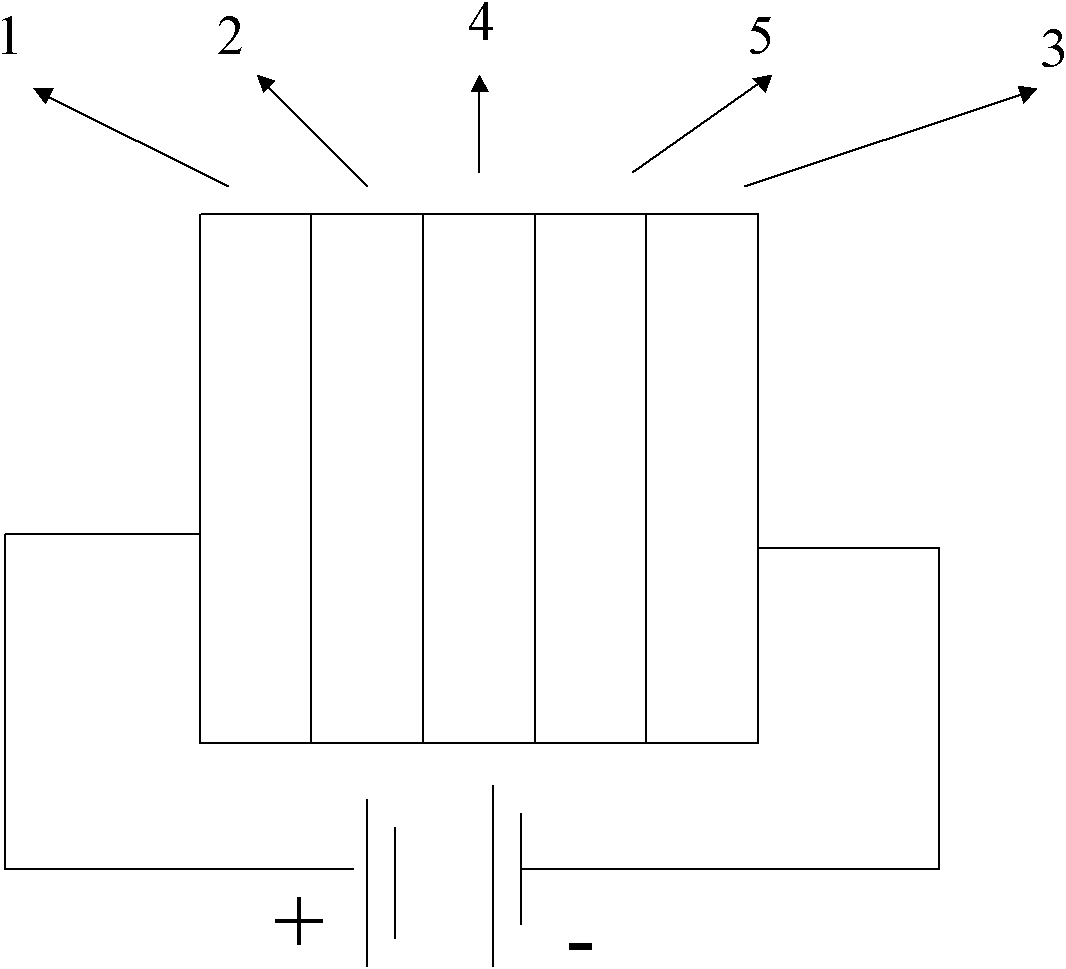
2-(2'-hydroxylphenyl)-benzothiazole derivative luminescent materials
A technology of hydroxyphenyl and benzothiazole, which is applied in the field of organic electroluminescent materials, can solve the problems of high driving voltage, difficult growth of single crystal, stagnation, etc., and achieve the effect of high reaction yield and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis of 2-(4'-dimethylamino-2'-methoxybenzene)-benzothiazole (P-1)
[0026]P-aminosalicylic acid (6g, 39.21mmol) and o-aminothiophenol (6mL, 55.68mmol) were mixed, and polyphosphoric acid (200mL) was used as solvent and dehydrating agent. Under nitrogen protection, heat in an oil bath with mechanical stirring at 150-160°C for 4 hours. After the reaction was cooled, it was poured into ice water to produce a large amount of yellow solid, which was filtered by suction. Add the solid to sodium bicarbonate aqueous solution to remove the remaining phosphoric acid, filter the off-white solid with column chromatography (silica gel, dichloromethane) to obtain the milky white pure product 2-(4'-amino-2'-hydroxyphenyl)-benzene And thiazole (P) 6.2g, yield 65.33%.
[0027] 2-(4'-amino-2'-hydroxybenzene)-benzothiazole (P) (1.33g, 5.496mmol), NaH (790mg, 32.917mmol), CH 3 I (2.05 mL, 32.915 mmol), THF (100 mL, dry) were added into a three-neck flask, and the reacti...
Embodiment 2
[0028] Example 2: Synthesis of 2-(4'-dimethylamino-2'-hydroxybenzene)-benzothiazole (P-2)
[0029] Mix P-1 (1.109g) and pyridine hydrochloride (anhydrous) thoroughly, and heat in an oil bath at 170-185°C for 2 hours under the protection of nitrogen. Add NaHCO after the reaction cools 3 The aqueous solution was freed from bubbles, extracted with dichloromethane, and evaporated to dryness under reduced pressure. The resulting solid was separated by column chromatography (silica gel, the volume ratio of dichloromethane / petroleum ether was 1:1) to obtain the product P-2 (744.9 mg, yield 71%) as light yellow powder. By-products are amino monosubstituents. The molecular ion mass determined by liquid mass analysis is: 271.1; theoretical element content (%) C 15 h 14 N 2 OS: C, 66.64; H, 10.36; N, 5.22, measured element content (%): C, 66.75; H, 10.34; N, 5.131. The above analysis results indicated that the obtained product was the expected product.
Embodiment 3
[0030] Example 3: Synthesis of 2-(4'-dianilino-2'-methoxybenzene)-benzothiazole (P-3)
[0031] (1) The basic molecule P (4g, 16.529mmol), phthalic anhydride (2.69g, 18.176mmol), triethylamine (4mL), xylene (200mL) were added to a three-necked flask, and the reaction mixture was decomposed under nitrogen protection. Heat to reflux for 9 hours. After stopping the reaction, the reaction mixture was suction filtered, washed with water several times, and then dried to obtain 5.99 g of milky white flocculent solid (PDoOH). The product PDoOH, dried potassium carbonate (3.33g, 24.130mmol), and 300mL acetonitrile (analytical grade untreated) were added into a one-necked bottle, and heated to reflux for 3 hours under nitrogen protection. After cooling, add CH 3 I (1.5mL, 24.1mmol), continue to heat under slow reflux for 10 hours. After the reaction, cool and filter with CH 2 Cl 2 Wash the filter cake, evaporate the filtrate to dryness under reduced pressure to obtain PDoMe as a mil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com