Method for synthesizing beta-(3,5-di-tert-butyl-4-hydroxyphenyl)methyl propionate
A technology of di-tert-butylphenol and hydroxyphenyl, which is applied in the field of synthesis of intermediate beta-methyl propionate, can solve the problems of increasing production cost, increasing cost, intensifying side reactions, etc., and improving product quality and yield , the effect of saving production costs and reducing reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
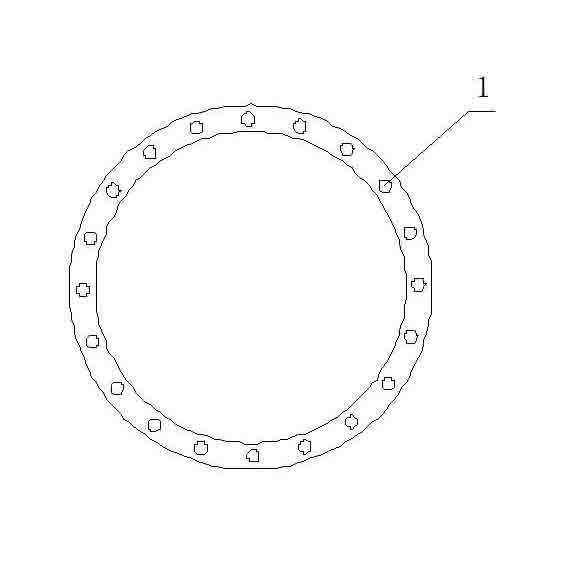
[0017] To the airtight with reflux condenser, mechanical stirring, trap, nitrogen gas line, vacuum line and inserted into the bottom of the reaction kettle, such as figure 1 with figure 2 Add 309 g (1.5 mol) of 2,6-di-tert-butylphenol into the 1-liter stainless steel reaction kettle of the circular dropper shown (number 1 in the figure is the drop hole), vacuumize, replace with nitrogen for more than 3 times, and give Heat the reactor to 55~60°C, continue vacuuming, add 3.71g (0.033mol, about 0.6% of the weight of 2,6-di-tert-butylphenol) 50% KOH aqueous solution under negative pressure, and start Stir, then continue to heat up to 90-115 ℃, remove the reaction system and the water generated by the reaction of the catalyst and 2.6-di-tert-butylphenol (subject to the fact that the water does not flow out) is collected in the cold trap, and the obtained Cool down the temperature of the slurry to 95°C, and start to add 131.6g (1.53mol) methyl acrylate dropwise into the reactor. ...
Embodiment 2
[0020] Add 400 g (1.94 mol) of 2,6- Di-tert-butylphenol, vacuumize, replace with nitrogen more than 3 times, heat the reactor to between 55°C and 60°C, continue vacuuming, and add 5g (0.045mol, weight about 2,6-di 0.625% of tert-butylphenol) 50% KOH aqueous solution, start stirring, and then continue to heat up to 90-110 ℃, remove the reaction system and the water generated by the reaction of the catalyst and 2.6-di-tert-butylphenol (water does not flow out ) collected in the cold trap, cooled the slurry obtained in the reactor to 95°C, and began to add 175g (2.03 mol) of methyl acrylate dropwise to the reactor, and controlled the rate of addition, and the dripping was completed within about 30 minutes. During the exothermic reaction, the temperature is controlled between 95-105°C. After the dropwise addition is completed, nitrogen gas is filled to a pressure of 0.4MP, and the temperature is kept between 95-105°C for 3 hours. Cool the system down to about 80°C, vacuum the sys...
Embodiment 3
[0023]Add 309 g (1.5 mol) of 2,6- Di-tert-butylphenol, vacuumize, replace with nitrogen more than 3 times, heat the reactor to 55~60°C, continue vacuuming, and add 6.18g (0.055mol, weight about 2,6- 1% of di-tert-butylphenol) 50% KOH aqueous solution, start stirring, and then continue to heat up to 90-115 ℃, remove the reaction system and the water generated by the reaction of the catalyst and 2.6-di-tert-butylphenol (water will come out flow) into the cold trap, lower the temperature of the slurry obtained in the reactor to 90°C, and start to add 135g (1.57mol) methyl acrylate dropwise to the reactor, and control the dropping rate, and the drop will be completed within about 30 minutes During the exothermic reaction, the temperature is controlled between 95-105°C during the dropwise addition. After the dropwise addition is completed, nitrogen gas is filled to a pressure of 0.6MP, and the temperature is kept between 90-100°C for 3 hours. Cool the reaction system to about 80°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com