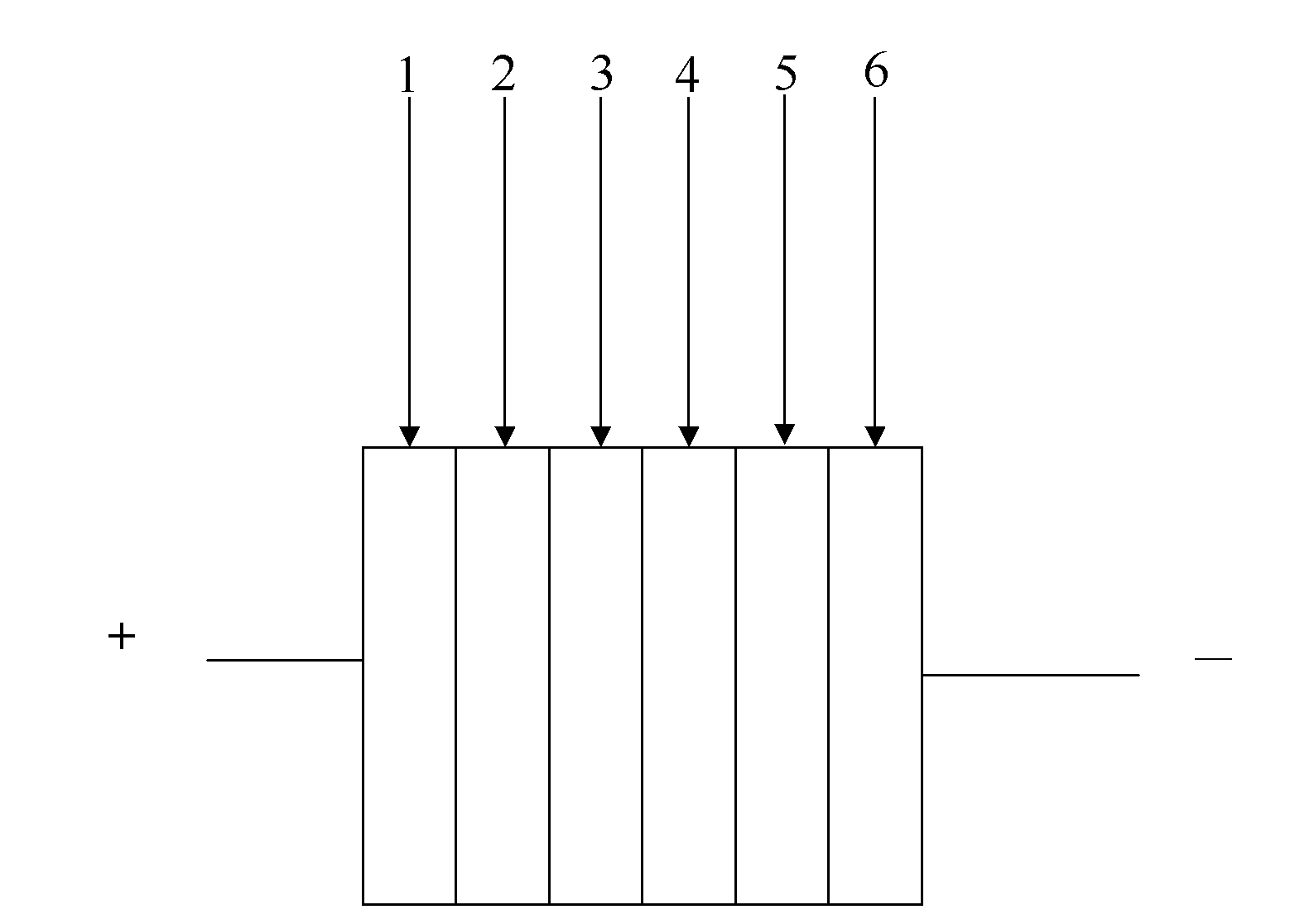
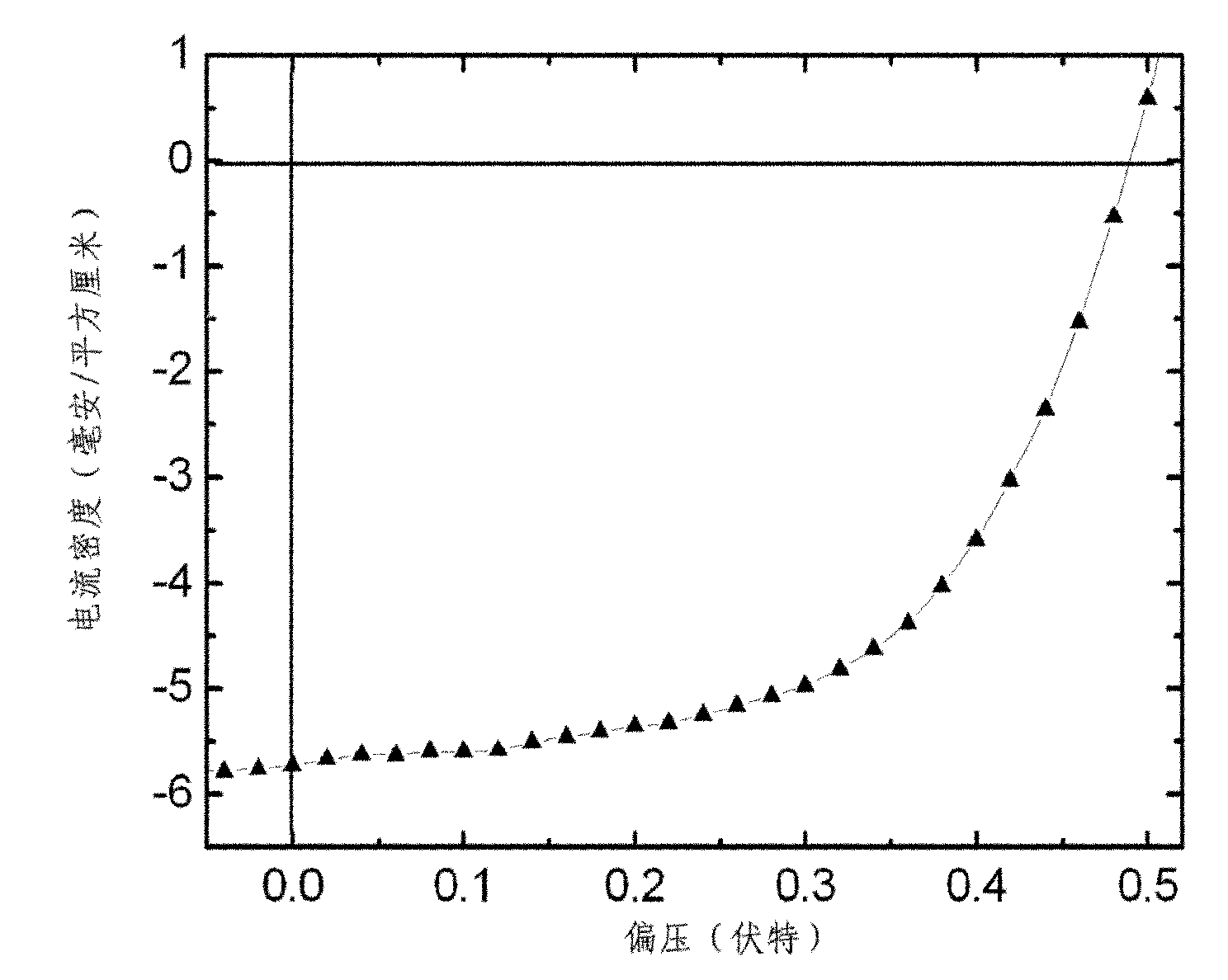
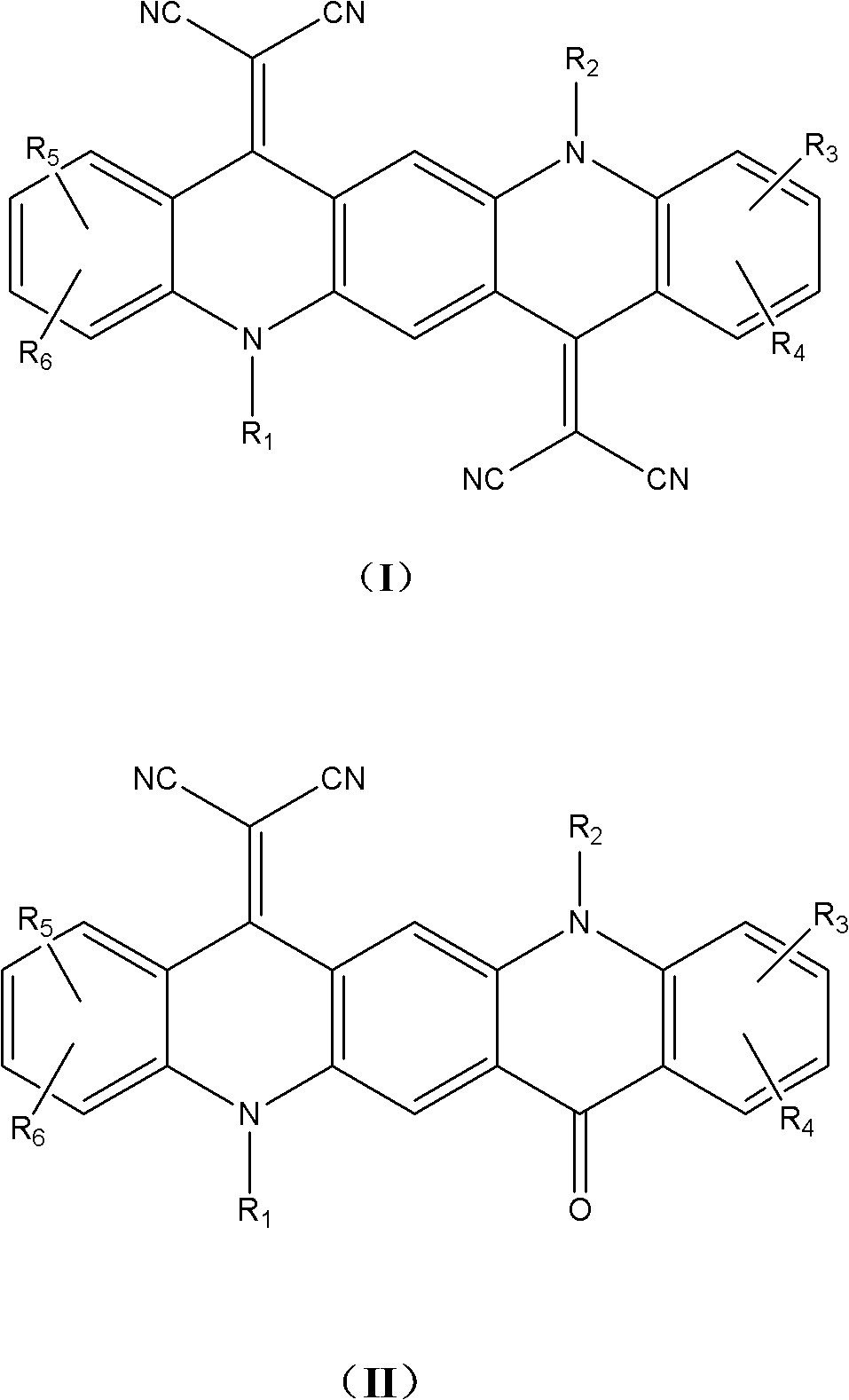
Nitrile substituted quinacridone compounds and application thereof in organic solar cell
A technology for solar cells and quinacridone, which is used in organic chemistry, circuits, photovoltaic power generation, etc., can solve the problems of limiting the light absorption efficiency and absorption range of devices, limiting the conversion efficiency of devices, and limiting wide application, etc., and achieves convenient purification, High fill factor, easily repeatable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the synthesis of DCN-4CTMQA
[0036] Under the protection of nitrogen, add 1 gram of 1,3,8,10-tetramethyl-5,12-dibutylquinacridone into 50ml of acetic anhydride, after completely dissolving, add four times the molar equivalent of propylene glycol Nitrile, heated to reflux for ten hours. The acetic anhydride was distilled off under reduced pressure, and the obtained solid was subjected to silica gel column chromatography with dichloromethane as the developing solvent to obtain 0.3 g of DCN-4CTMQA with a yield of 27.2%. Mass spectrum molecular ion peak: 577.6. Elemental Analysis: C 38 h 36 N 6 , Theoretical value: C, 79.14; H, 6.29; N, 14.57, measured: C, 79.26; H, 6.31, N, 14.43.
[0037]
Embodiment 2
[0038] Embodiment 2: the synthesis of DCN-6CTMQA
[0039] The synthesis method of this compound is similar to that of DCN-4CTMQA, except that n-bromohexane is used in the alkylation of quinacridone. The molecular ion peak of the product: 632.36. Elemental Analysis: C 42 h 44 N 6 , Theoretical value: C, 79.71; H, 7.01; N, 13.28, measured: C, 79.74; H, 7.05; N, 13.21.
[0040]
Embodiment 3
[0041] Embodiment 3: the synthesis of DCN-8CTMQA
[0042] The synthesis method of this compound is similar to DCN-4CTMQA except that n-bromooctane is used in the alkylation of quinacridone. The molecular ion peak of the product: 688.43. Elemental Analysis: C 46 h 52 N 6 , Theoretical value: C, 80.19; H, 7.61; N, 12.20, measured: C, 80.21; H, 7.63; N, 12.16.
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com