Method for preparing copper calcium titanate ceramic
A technology of copper calcium titanate and ceramics, which is applied in the field of preparation of copper calcium titanate ceramic powder based on the improved co-precipitation method, can solve the problems of difficult control of pH value adjustment and many raw materials, and achieve less impurity phases, simple preparation process, The effect of stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
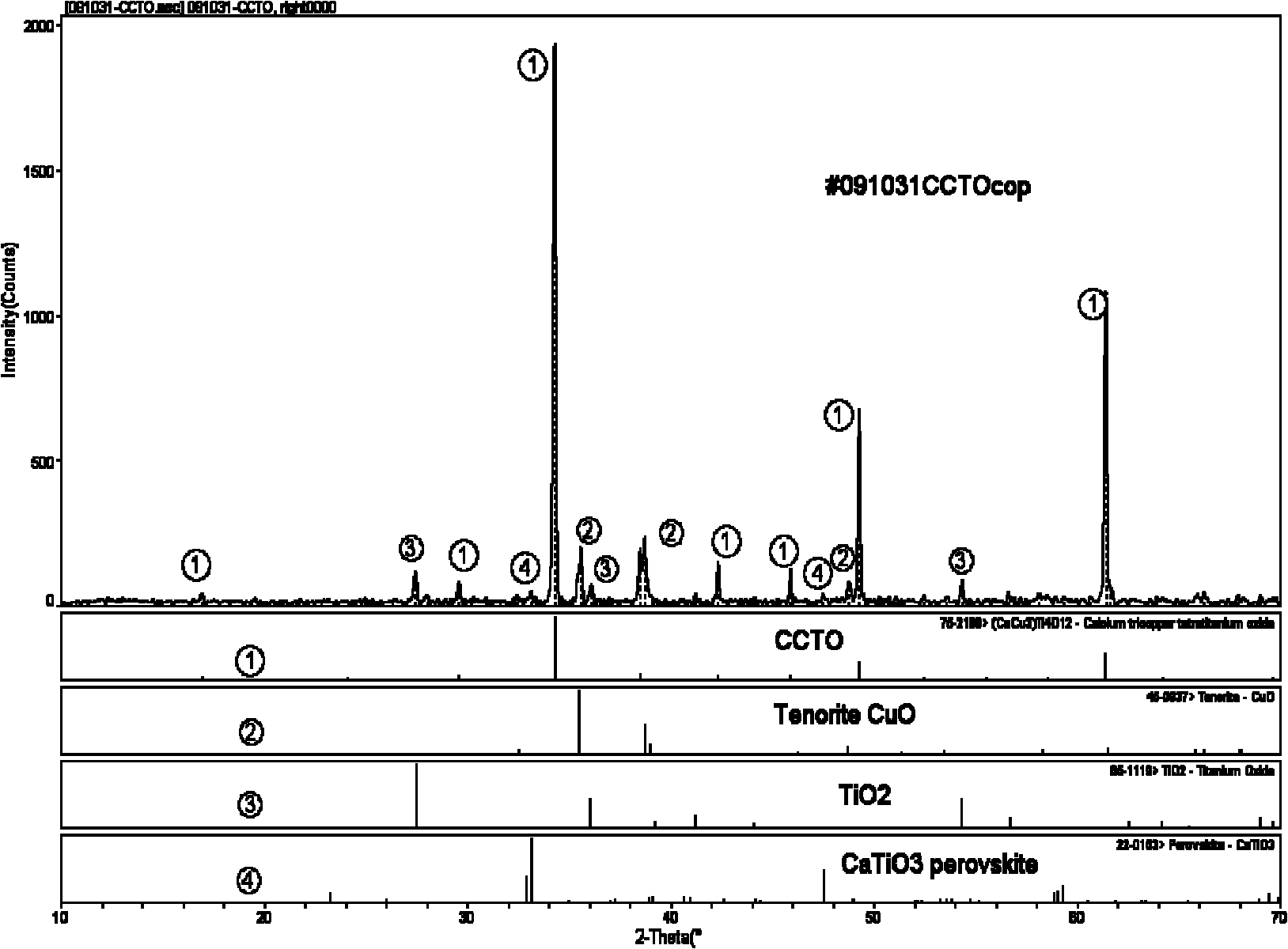
Embodiment 1
[0029] 1) 0.1mol tetrabutyl titanate Ti(O 4 h 9 ) 4 Dissolve in 250ml absolute ethanol to make A solution, add 0.2mol oxalic acid H 2 C 2 o 4 2H 2 Dissolve O in 750ml of deionized water to make solution B. The two solutions of A and B are mixed and recorded as HTO solution. A white precipitate is formed after a reaction. Stir with a stirrer to make the solution clear again;
[0030] 2) Add 0.025mol calcium nitrate Ca(NO 3 ) 2 ·H 2 O, 0.075mol copper nitrate Cu(NO 3 ) 2 ·3H 2 Dissolve O together in 1000ml deionized water and dissolve completely to prepare calcium and copper solution C;
[0031] 3) ammonium acetate CH 3 COONH 4 Dissolve in deionized water, prepare 400ml of ammonium acetate solution with a solubility of 1mol / L, as buffer D;
[0032] 4) Slowly add the calcium copper solution C into the HTO solution of the mixed solution of A and B, and at the same time, drop the ammonium acetate solution D evenly, so that the pH value of the reaction solution is kept...
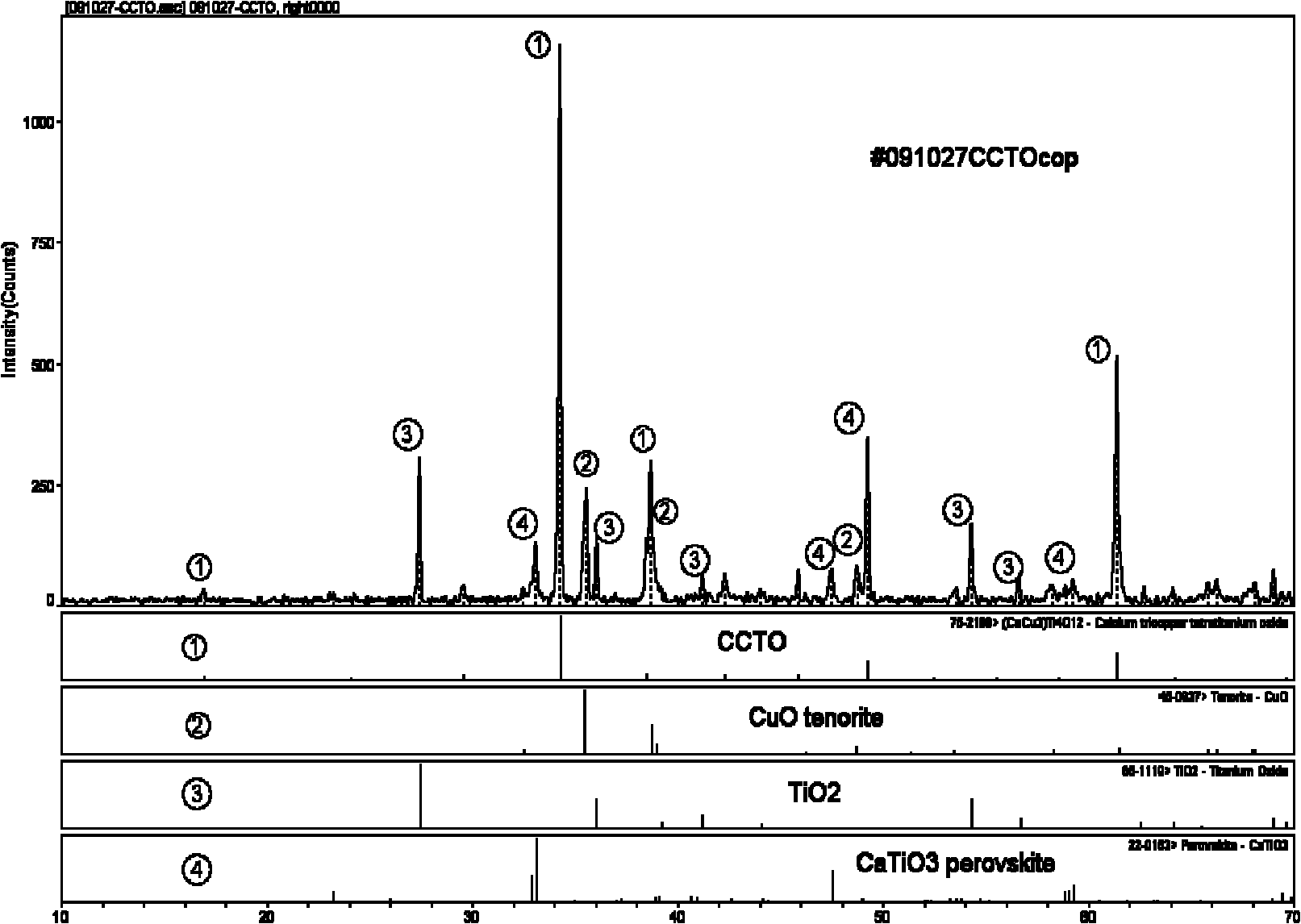
Embodiment 2
[0036] 1) 0.1mol tetrabutyl titanate Ti(O 4 h 9 ) 4 Dissolve in 250ml absolute ethanol to make A solution, add 0.2mol oxalic acid H 2 C 2 o 4 2H 2 Dissolve O in 750ml of deionized water to make solution B. The two solutions of A and B are mixed and recorded as HTO solution. A white precipitate is formed after a reaction. Stir with a stirrer to make the solution clear again;
[0037] 2) Add 0.025mol calcium nitrate Ca(NO 3 ) 2 ·H 2 O, 0.075mol copper nitrate Cu(NO 3 ) 2 ·3H 2 Dissolve O together in 1000ml deionized water and dissolve completely to prepare calcium and copper solution C;
[0038] 3) Dissolve nitric acid-ammonium nitrate in deionized water, prepare 400ml of nitric acid-ammonium nitrate with a solubility of 1mol / L, and add it as buffer D into the stirred and clarified mixed liquid A and B HTO solution.
[0039] 4) Slowly add the calcium copper solution C into the HTO solution of the mixed solution of A and B, and no precipitation is seen. At this time, ...
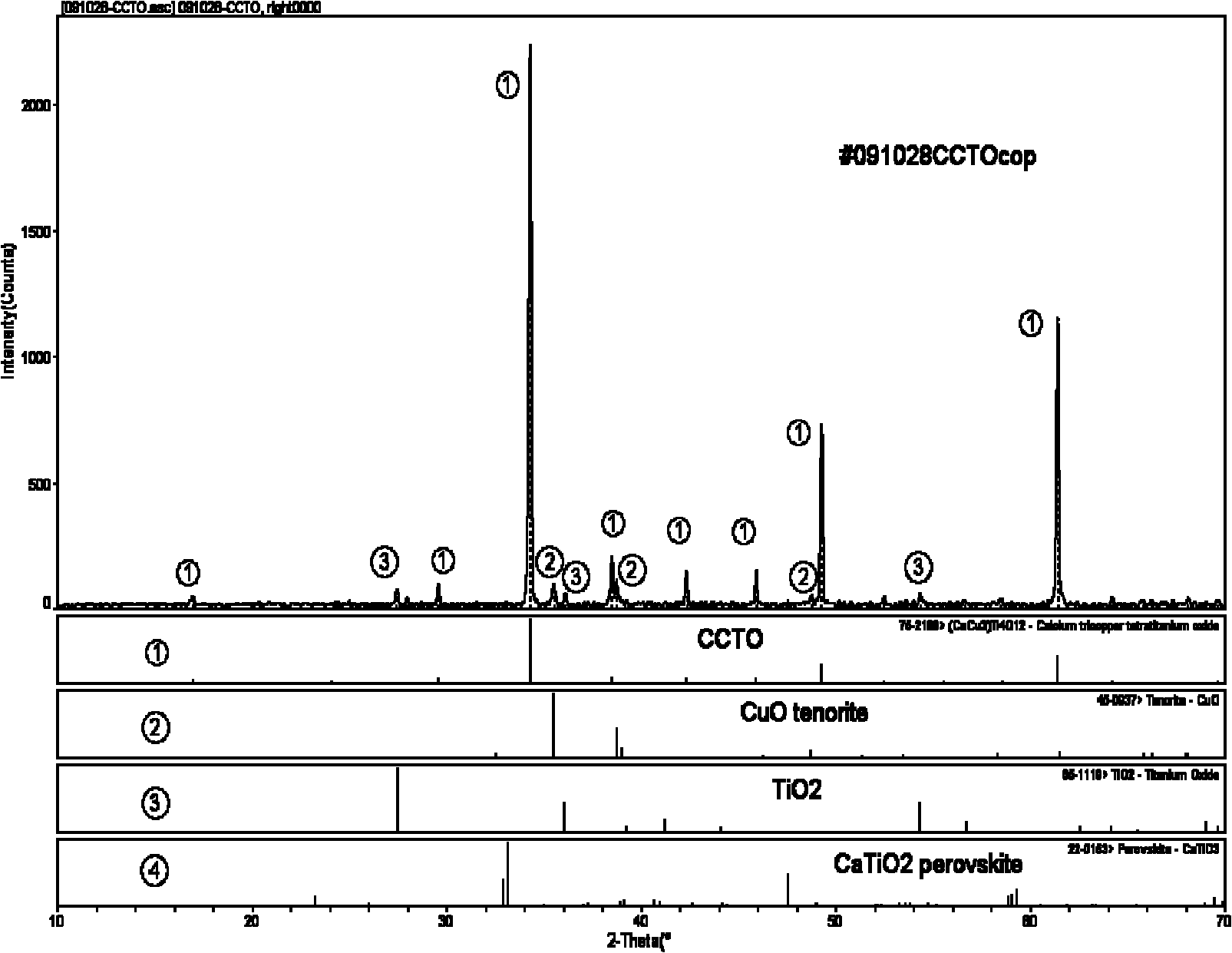
Embodiment 3
[0043] 1) 0.1mol tetrabutyl titanate Ti(O 4 h 9 ) 4Dissolve in 250ml absolute ethanol to make A solution, add 0.2mol oxalic acid H 2 C 2 o 4 2·H 2 Dissolve O in 750ml of deionized water to make solution B. The two solutions of A and B are mixed and recorded as HTO solution. A white precipitate is formed after a reaction. Stir with a stirrer to make the solution clear again;
[0044] 2) Add 0.025mol calcium nitrate Ca(NO 3 ) 2 ·H 2 O, 0.075mol copper nitrate Cu(NO 3 ) 2 ·3H 2 Dissolve O together in 1000ml deionized water and dissolve completely to prepare calcium and copper solution C;
[0045] 3) ammonium acetate CH 3 COONH 4 Dissolve in deionized water, prepare 400ml of ammonium acetate solution with a solubility of 1mol / L, as buffer D;
[0046] 4) Slowly add the calcium copper solution C into the HTO solution of the mixed solution of A and B, and at the same time drop evenly the ammonium acetate solution D, so that the pH value of the reaction solution is kept ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| loss value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com