Pyridine quaternary ammonium salt polyurethane and preparation method thereof
A technology of pyridinium quaternary ammonium salt and polyurethane, which is applied in the field of pyridinium quaternary ammonium salt polyurethane and its preparation, which can solve the problems of limited application fields, poor stability and antibacterial properties of water-based polyurethane, and achieve improved stability, enhanced antibacterial activity, and stability and strong antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
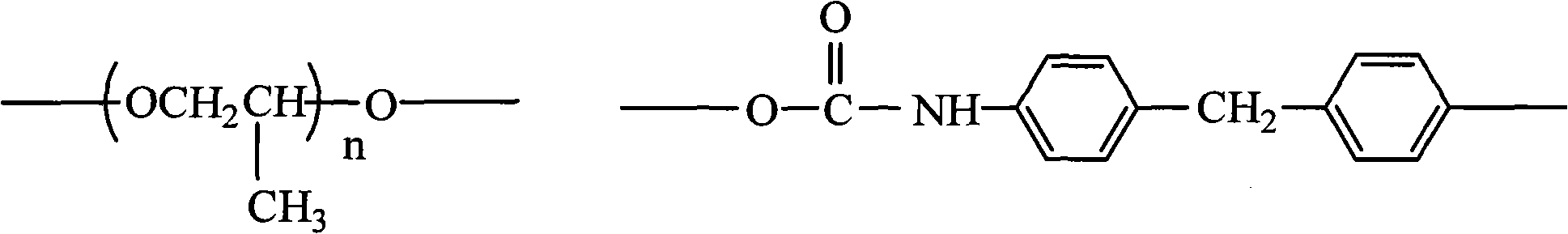
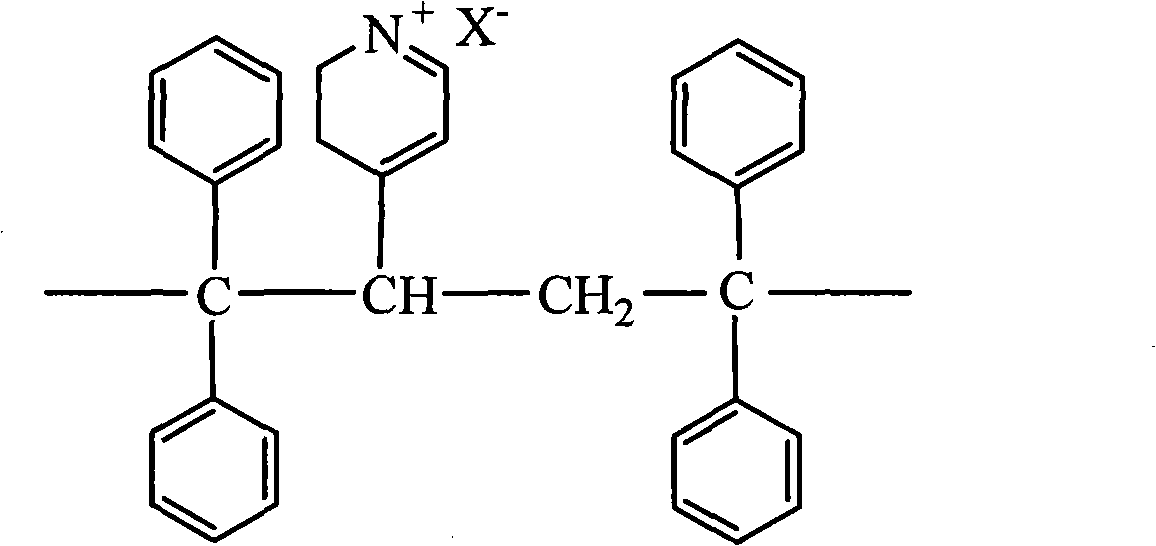
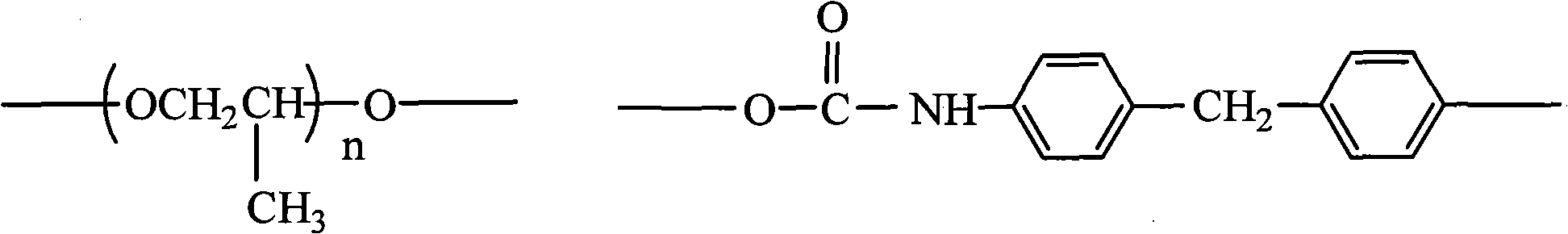
[0030] The present invention also provides a kind of preparation method of pyridinium quaternary ammonium salt polyurethane, comprises the following steps:
[0031] 1) mixing polypropylene glycol (PPG) with diphenylmethane diisocyanate (MDI), and performing a condensation reaction to obtain an isocyanate group-terminated prepolymer 1;
[0032] 2) Add tetraphenylethylene glycol (TPED) to the isocyanate group-terminated prepolymer 1 obtained in step 1), and react in the presence of a catalyst to obtain a benzene ring-containing prepolymer 2;
[0033] 3) Mix the benzene ring-containing prepolymer 2 obtained in step 2) with vinylpyridine in proportion, and react with the solvent to obtain the pyridine group-containing prepolymer 3;
[0034] 4) adding halogenated hydrocarbon or hydrohalic acid to the pyridine group-containing prepolymer 3 obtained in step 3), to obtain the pyridine quaternary ammonium salt polyurethane.
[0035] The preparation mechanism of the pyridine quaternary...
Embodiment 1
[0053] (1) Add 120 g of PPG (molecular weight 2000) and 20 g of MDI into a 500 mL four-neck flask, heat to 75° C. for 3 hours under the protection of nitrogen, and cool to 25° C. to obtain product A1.
[0054] (2) Add 175g of product A, 15g of TPED, 0.5g of dibutyltin dilaurate and 180mL of MEK into a 500mL four-neck flask, and stir at a constant temperature of 25°C for 24 hours. The obtained product was repeatedly suction-filtered three times with methanol as a solvent, and vacuum-dried at 4° C. to obtain product B1.
[0055] (3) Add 100 g of product B1 into a 500 mL four-neck flask, add DMF 10 g and vinylpyridine 10 g in turn under nitrogen protection, and then place it in a constant temperature water bath at 75°C for 5 hours to obtain product C1.
[0056] (4) Add 25 g of product C1 into a 500 mL four-neck flask, add 2.0 g of hydrobromic acid, and perform quaternization reaction for 24 hours. Evaporate with a rotary evaporator until the volume of the residue is 30%, and dry...
Embodiment 2
[0059] (1) Add 100 g of PPG (molecular weight: 2000) and 25 g of MDI into a 500 mL four-necked flask, and heat to 75° C. for 3 hours under the protection of nitrogen; cool to 25° C. to obtain product A2.
[0060] (2) Add 275g of reaction product A, 14.65g of TPED, 0.5g of stannous octoate and 180mL of MEK into a 500mL four-neck flask, and stir at a constant temperature of 25°C for 24 hours. The obtained product was repeatedly suction-filtered three times with methanol as a solvent, and vacuum-dried at 4° C. to obtain product B2.
[0061] (3) Add 100 g of product B2 to a 500 mL four-neck flask, add DMF 10 g and vinylpyridine 10 g in sequence under nitrogen protection, and then place it in a constant temperature water bath at 75°C for 5 hours to obtain product C2.
[0062] (4) Add 25 g of the product C2 into a 500 mL four-neck flask, add 2.0 g of hydrobromic acid, and perform quaternization for 24 hours. Evaporate with a rotary evaporator until the volume of the residue is 20%,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com