Method for preparing etoposide nanometer suspension freeze-drying preparation
A freeze-dried preparation, the technology of Poside Nanometer, which is applied in the field of medicine, can solve the problems of serious sensitization reaction, high can only reach 0.5%, toxic and side effects in clinical application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
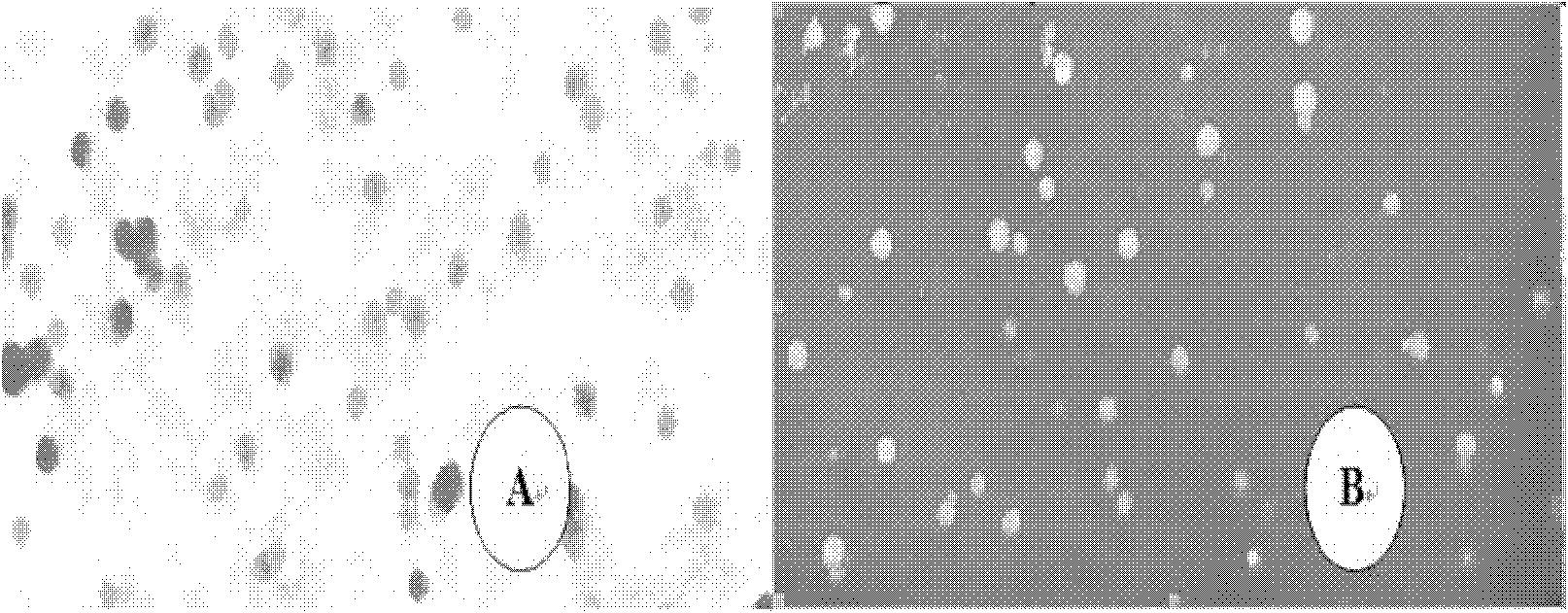
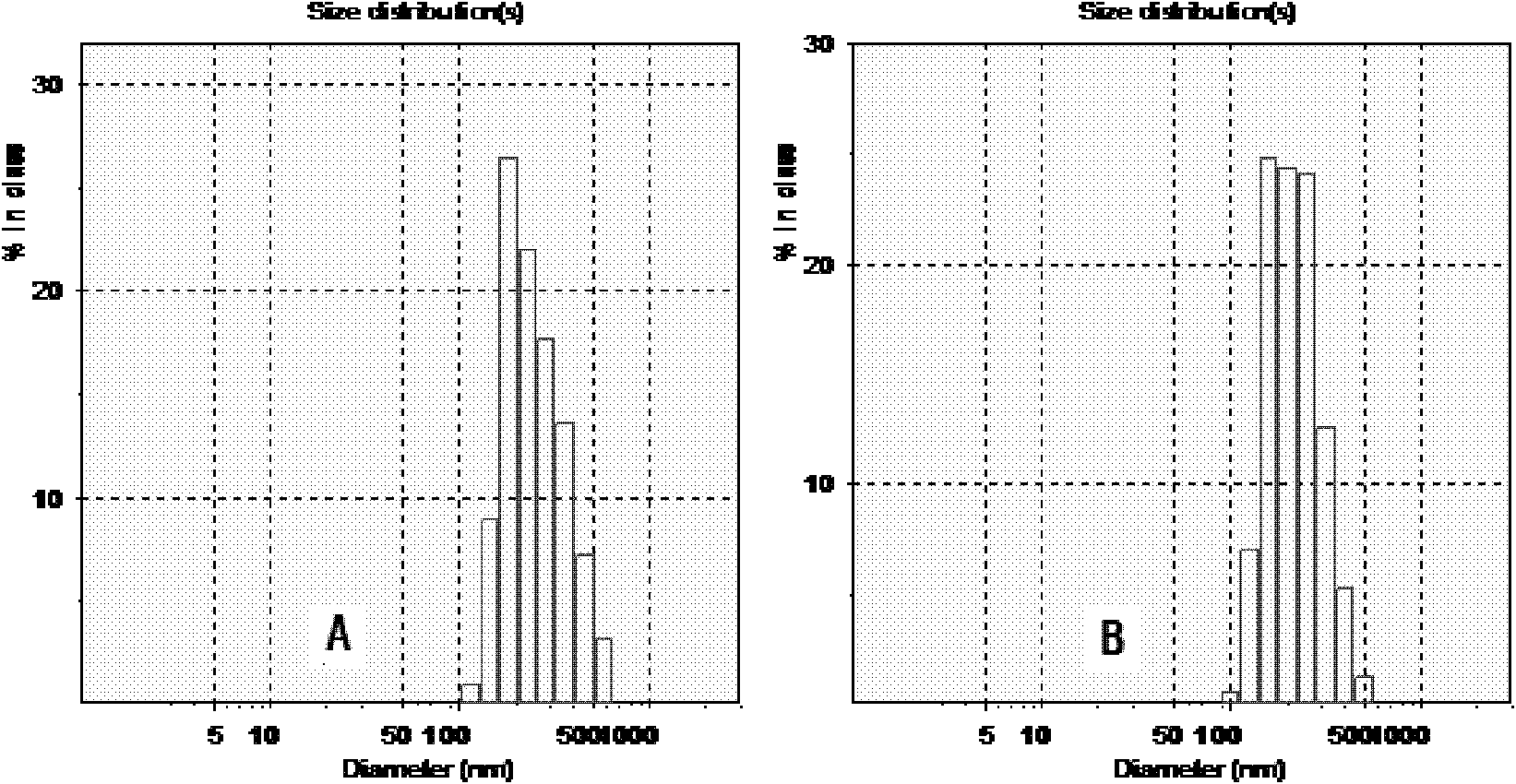
[0031] 10 mg of etoposide was dissolved in 1.0 ml of methylene chloride, and added to 5 ml of a 2% (W / V) aqueous solution of human serum albumin, and the mixture was sheared at high speed (9000 rpm) for 3 min to form colostrum, which was then Transfer to the ultrasonic treatment tank, ultrasonic (600W) 5min, the obtained suspension was evaporated under reduced pressure at 40°C for 15-20min to remove dichloromethane, and the etoposide nanosuspension was obtained, and its particle size range was 150 ~300nm.
[0032] The above-prepared suspension was divided into vials, pre-frozen in a low-temperature refrigerator (-80°C) for 24 hours, and then transferred to a freeze dryer (-40°C, 0.5mbar) for 48 hours to obtain etoposide nanomixtures. Suspension freeze-dried formulations.
Embodiment 2
[0034] Dissolve 90 mg of etoposide in 3.0 ml of chloroform, and add it to 30 ml of a 1% (W / V) aqueous solution of human serum albumin. The mixture is sheared at high speed (12000 rpm) for 5 min, and then transferred to a high-pressure milk In the homogenizer, 1500bar was circulated for 20 times, and the obtained suspension was evaporated at 40°C under reduced pressure for 15-20 minutes to remove chloroform. The obtained suspension was translucent and the particle diameter was 160-220nm.
[0035] The above-prepared suspension was divided into vials, pre-frozen in a low-temperature refrigerator (-80°C) for 24 hours, and then transferred to a freeze dryer (-40°C, 0.5mbar) for 48 hours to obtain etoposide nanomixtures. Suspension freeze-dried formulations.
Embodiment 3
[0037] Dissolve 200 mg of etoposide in a mixed solution of 3.0 ml of chloroform and 0.4 ml of absolute ethanol, add to 100 ml of bovine serum albumin aqueous solution with a concentration of 3% (W / V), shear (9000 rpm) for 2 min, and transfer rapidly Put it in a high-pressure milk homogenizer, circulate 15 times at 1300 bar, remove the organic solvent quickly from the obtained suspension under reduced pressure at 35°C, and obtain etoposide albumin nanosuspension, the particle size range of which is 300-450nm .
[0038]The above-prepared suspension was divided into vials, pre-frozen in a low-temperature refrigerator (-80°C) for 24 hours, and then transferred to a freeze dryer (-40°C, 0.5mbar) for 48 hours to obtain etoposide nanomixtures. Suspension freeze-dried formulations. After adding physiological saline, it can quickly return to the state before freeze-drying, and the particle size of the particles does not change.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com