Surfactant containing adamantane and preparation method thereof
A technology of surfactant and adamantane, which is applied in the field of surfactant and its preparation, can solve the problems that have not been reported, and achieve the effects of good lipophilicity, high product yield and purity, and high biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
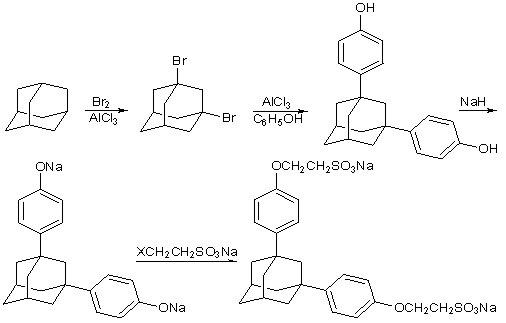
[0027] Synthesis of 1,3-dibromoadamantane: In a 150ml three-neck flask equipped with a reflux condensation and tail gas absorption device, add 20g (0.147mol) of adamantane, 20ml of dichloromethane, and 0.8g (0.014mol) of iron powder, and feed Nitrogen, under the temperature condition of 0~5℃, slowly add 18ml bromine (54g, 0.34mol) dropwise while stirring, then react at the temperature condition of 15~25℃ for 8 hours, add saturated sodium bisulfite solution and stir well, remove Excess bromine, the resulting solid was washed with water, dried, and recrystallized from methanol / water to obtain 32.4 g of 1,3-dibromoadamantane, with a yield of 75.0%.
[0028] Synthesis of 1,3-di(4-hydroxyphenyl)adamantane: In a 150ml reaction bottle equipped with reflux condensation and tail gas absorption device, add 6g (20.4mmol) of dried 1,3-dibromoadamantane, phenol 40g (0.425mol), heated to 80-85°C, added 1.36g (10.2mmol) of aluminum trichloride, reacted for 10 hours, washed with hot water to ...
Embodiment 2
[0032] The synthesis steps of 1,3-dibromoadamantane and 1,3-bis(4-hydroxyphenyl)adamantane are shown in Example 1.
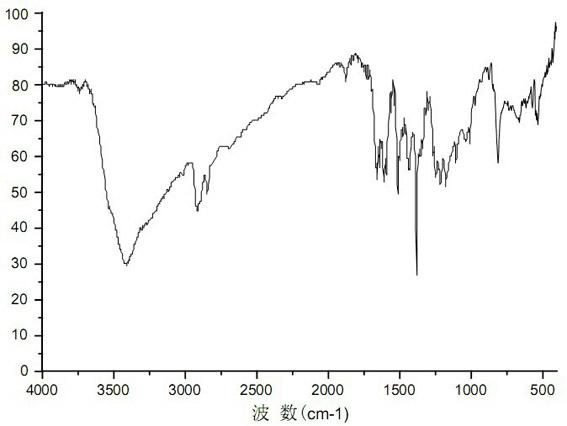
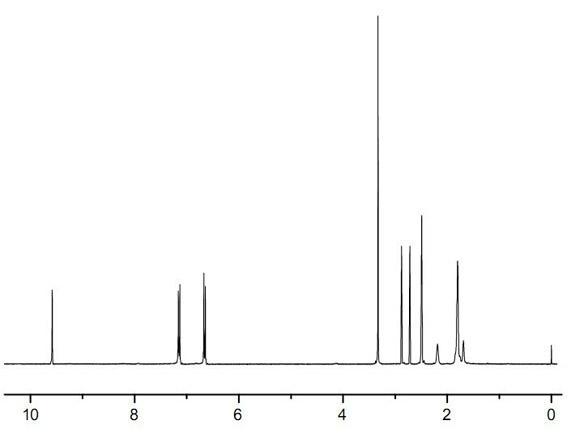
[0033] Synthesis of adamantane-1,3-diphenethyl ether sulfonic acid: Add 0.80 g (2.5 mmol) of 1,3-bis(4-hydroxyphenyl) adamantane and anhydrous N to a 150 ml three-necked flask equipped with a reflux condenser , N-dimethylformamide 25ml, stir until completely dissolved, add sodium hydroxide 0.40g (10mmol), slowly heat up to 50°C while stirring and maintain for 2 hours, pass through N 2 protection, and slowly added 1.16 g (5.5 mmol) of sodium 2-bromoethylsulfonate. Then heat up to 80°C to continue the reaction for 5 hours, add 100ml of water, stir for a while, filter to remove insoluble impurities, acidify to pH 4 with hydrochloric acid to precipitate a large amount of white solid, filter with suction, and dry the resulting solid at 45°C for 12 hours to constant temperature. 1.50 g was obtained, and 1.05 g of white flaky crystals were obtained after recrystalliza...
Embodiment 3
[0036] Synthesis of adamantane-1,3-diphenylethyl ether sulfonic acid: add anhydrous N,N-dimethylformamide 50ml to a 150ml three-necked flask equipped with a reflux condenser, N 2 After replacement, add 1.60 g (40 mmol) of sodium hydride, stir evenly, add 3.20 g (10 mmol) of 1,3-bis(4-hydroxyphenyl)adamantane, slowly raise the temperature to 40°C while stirring and keep it for 1 hour, and pass through N 2 After protection, until no bubbles continue to be generated, 5.25 g (25 mmol) of sodium 2-bromoethylsulfonate was added slowly, and the addition was completed in 1 hour. Then heat up to 60°C to continue the reaction for 4 hours, add 100ml of water, stir for a while, filter to remove insoluble impurities, acidify with hydrochloric acid to pH 4, precipitate a large amount of white solid, filter with suction, dry the resulting solid at 45°C for 12 hours to constant weight , 7.15g was obtained, and 4.73g of white flaky crystals were obtained after methanol / water recrystallization,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com