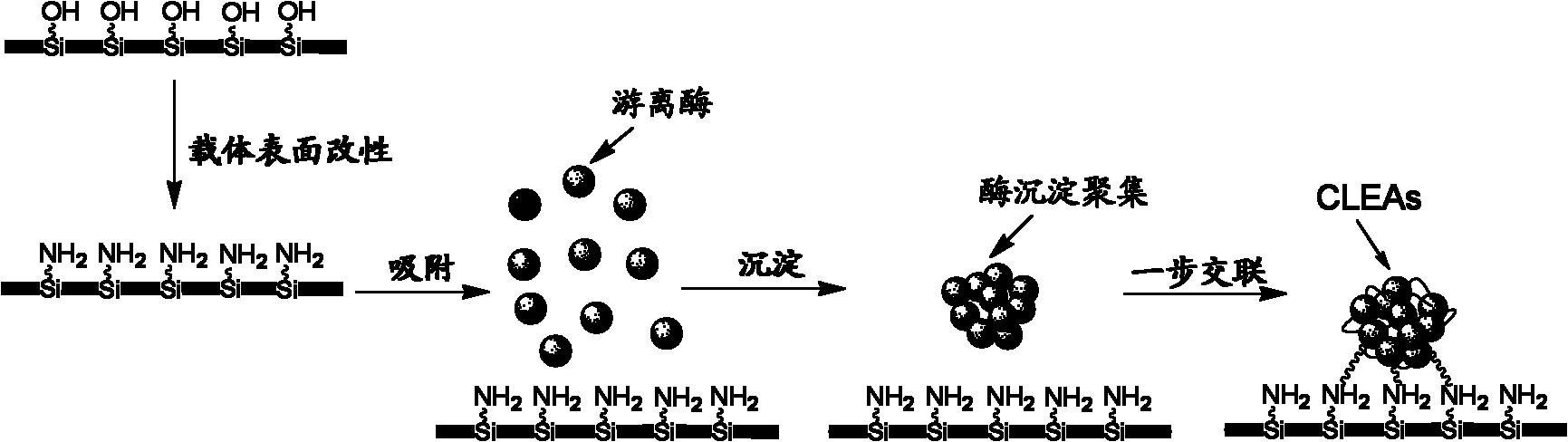
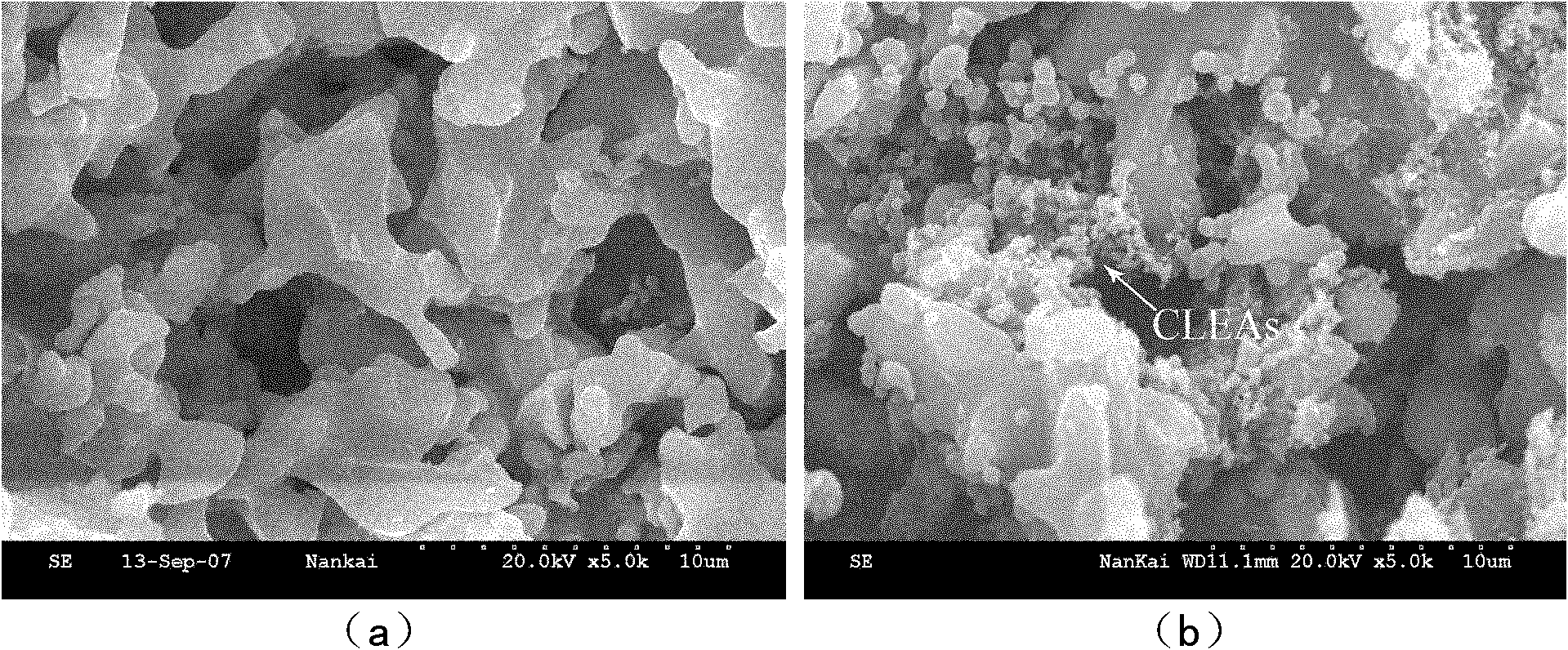
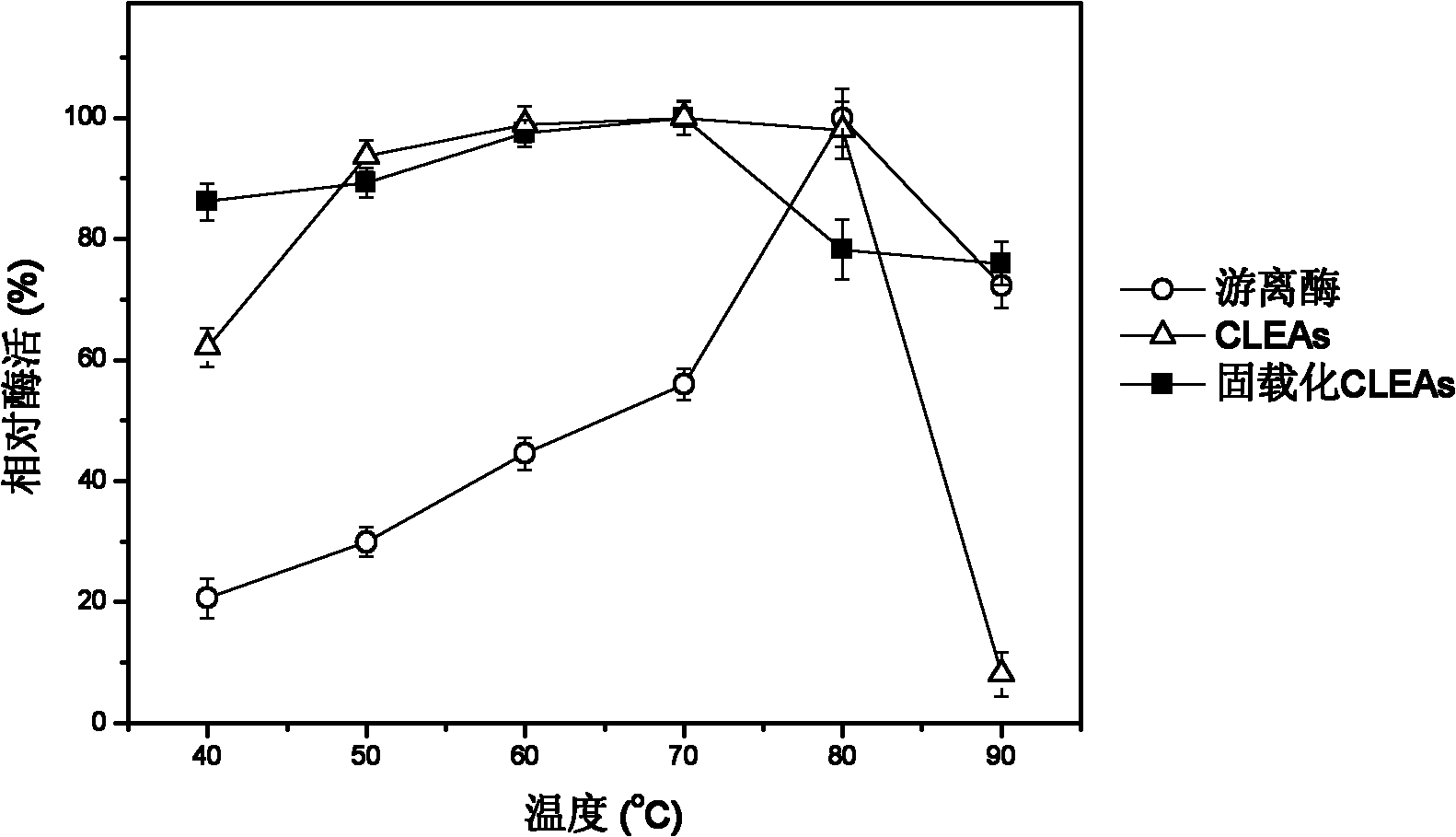
Macropore carrier 'synchronization method' covalent crosslinking-immobilized papain polymer and method
A technology of papain and covalent cross-linking, which is applied in the field of preparation of new immobilized enzyme biocatalysts, can solve the problems of chitosan carrier instability, unfavorable repeated recycling, low enzyme loading, etc., to overcome the unfavorable industrial Application, improvement of industrialization defects, simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Take 2g of macroporous silica gel (average pore diameter 1 μm, average particle diameter of carrier particles 5 mm, average porosity 0.6), wash and dry at 100° C. for 12 h until the mass does not change. According to the mass ratio of macroporous silica gel to silane coupling agent volume ratio of 1:5 (g:mL), take 10mL of γ-aminopropyltriethoxysilane and 20mL of absolute ethanol and mix it for 10min, then add 2g of dried macroporous silica gel carrier. Rotary evaporation under reduced pressure at 50°C until the liquid was no longer reduced, the carrier was washed three times with absolute ethanol, and dried at 100°C to obtain an amino-modified macroporous silica gel carrier, the surface amino content was measured to be 8.5 μmol / g. Dissolve papain in 0.2mol / L phosphate buffer (pH7.0) to prepare 4mL of 0.15g / mL enzyme solution, add 2g of amino-modified macroporous silica gel carrier, and shake at 25°C for 3.5h . The carrier adsorbed with papain was taken out, washed once...
Embodiment 2
[0039] Take 6g of macroporous aluminum oxide (average pore diameter 0.8 μm, average particle diameter of carrier particles 5 mm, average porosity 0.5), wash it, and dry it at 120° C. for 12 hours until the mass does not change. Take 20mL of N-(β-aminoethyl)-γ-aminopropyltrimethoxysilane and mix it with 60mL of absolute ethanol for 20min according to the mass ratio of macroporous silica gel to silane coupling agent volume ratio of 1:3.3 (g:mL). Finally, 6 g of dried macroporous aluminum oxide carrier was added. Rotary steam under reduced pressure at 60°C until the liquid no longer decreases, wash the carrier four times with absolute ethanol, and dry at 120°C to obtain an amino-modified macroporous alumina carrier, the surface amino content is measured to be 10 μmol / g . Dissolve papain in 0.1 mol / L phosphate buffer (pH 4.0) to prepare 3 mL of 0.25 g / mL enzyme solution, add 3 g of amino-modified macroporous alumina carrier, shake at 4 °C Shake for 6 hours. The carrier adsorbed...
Embodiment 3
[0041] Take 10 g of macroporous cellulose particles (average pore diameter 1 μm, average carrier particle diameter 5 mm, average porosity 0.6), wash them, and dry them at 90° C. for 24 h until the mass does not change. Take 30mL of N-β-(aminoethyl)-γ-aminopropylmethyldimethoxysilane and mix it with 30mL of absolute ethanol according to the mass ratio of macroporous silica gel to silane coupling agent volume ratio of 1:3 (g:mL). After 30 minutes, 10 g of dried macroporous cellulose carrier was added. Rotary evaporation under reduced pressure at 70°C until the liquid was no longer reduced, the carrier was washed three times with absolute ethanol, and dried at 90°C to obtain an amino-modified macroporous cellulose carrier. The surface amino content was measured to be 8.7 μmol / g. Dissolve papain in 0.2mol / L phosphate buffer (pH10.0) to prepare 5mL of 0.05g / mL enzyme solution, add 5g of amino-modified macroporous cellulose carrier, shake at 15°C for 5h . The carrier adsorbed with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com