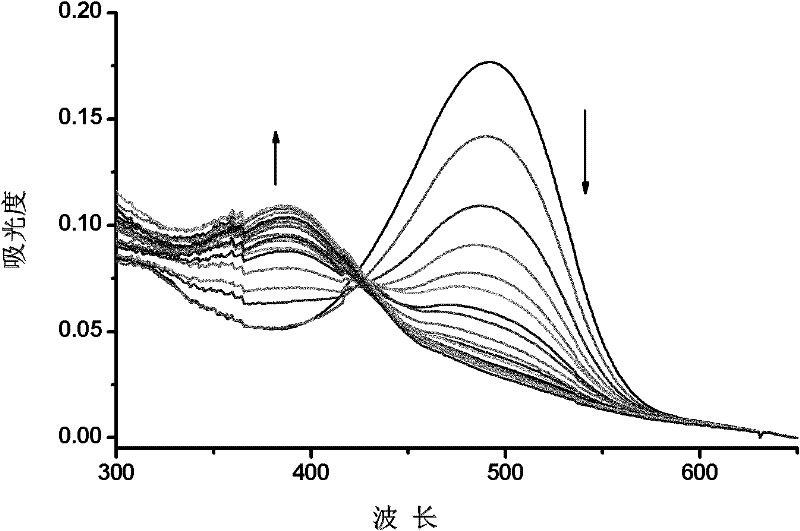
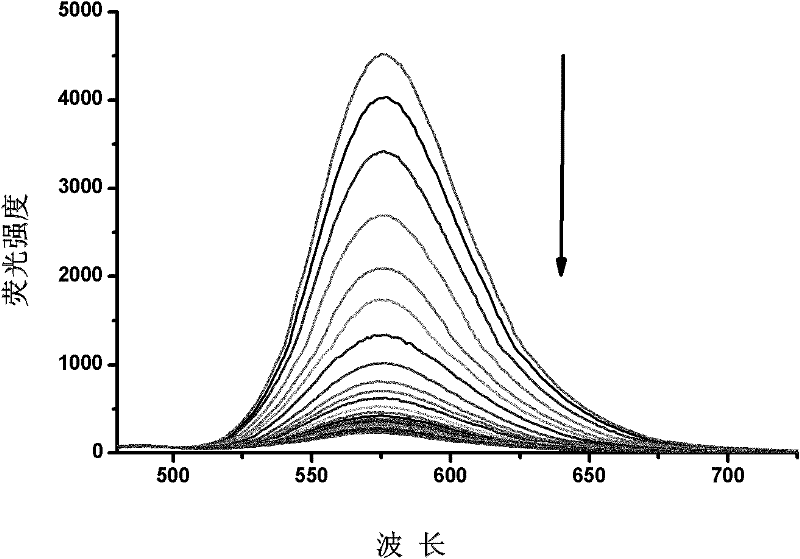
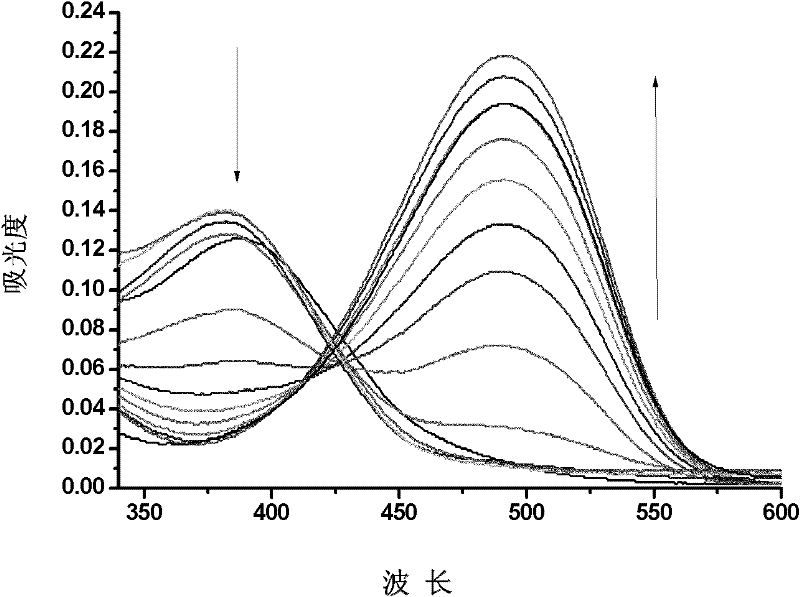
Fluorescence chemical sensor and preparation method and application thereof
A chemical sensor and fluorescence technology, applied in the field of chemical sensors, can solve the problems of reduced sensitivity, reduced host-guest interaction, unable to effectively detect cyanide ions, etc., to achieve simple detection and improve application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, preparation of fluorescent chemical sensor (structural formula shown in formula (IV))
[0033]
[0034] (1) Weigh 180 mg of chloromethylpyridine hydrochloride and 700 mg of aniline into a reaction flask, use sodium hydroxide solution as a solvent, add hexadecyl ammonium bromide as a phase transfer catalyst, heat to 45 ° C and stir for 48 hours, Extracted with dichloromethane and separated by column chromatography (ethyl acetate / petroleum ether=3:1) to obtain pure pale yellow 4'-(N,N-dimethylpyridylamino)benzene. Slowly add 1ml of phosphorus oxychloride dropwise to anhydrous DMF sealed reaction bottle in ice bath, stir at 0°C for 30 minutes, then add 500mg of 4'-(N,N-dimethylpyridylamino) prepared above Benzene, heated to 45°C and stirred for 72 hours. After the reaction, the system was naturally cooled to room temperature, added distilled water and stirred for 30 minutes, adjusted the pH value to 10 with potassium carbonate, stirred for 30 minutes, ext...
Embodiment 2
[0046] Embodiment 2, preparation of fluorescent chemical sensor (structural formula shown in formula (VI))
[0047]
[0048] (1) The preparation method of 4'-(N,N-dimethylpyridylamino)benzaldehyde is the same as that in Example 1, wherein the temperature of the oxidation reaction is 90°C, and the time of the oxidation reaction is 24 hours.
[0049] (2) Weigh 300mg 2-methylbenzothiazole and 300mg methyl iodide into the reaction flask, use chloroform as a solvent, heat to 80°C and stir for 48 hours, a precipitate is formed, after the reaction stops, naturally cool to room temperature, filter Washing obtains light yellow 2-methyl-N-methylbenzothiazole iodide salt (its structural formula is shown in formula (III), wherein, R 1 for S, R 2 is methyl);
[0050] (3) Weigh 150mg 2-methyl-N-methylbenzothiazole iodine salt and 500mg 4'-(N,N-dimethylpyridylamino)benzaldehyde (wherein, 2-methyl-N- The molar ratio of methylbenzothiazole iodide salt and 4'-(N,N-dimethylpyridylamino)ben...
Embodiment 3
[0053] Embodiment 3, preparation of fluorescent chemical sensor (structural formula shown in formula (VIII))
[0054]
[0055] (1) The preparation method of 4'-(N,N-dimethylpyridylamino)benzaldehyde is the same as in Example 1, wherein the temperature of the oxidation reaction is 65°C, and the time of the oxidation reaction is 48 hours.
[0056] (2) 2-Methyl-N-methylbenzothiazole iodine salt The method is the same as that in Example 2.
[0057] (3) The preparation method of trans 2-[4'-(N,N-dimethylpyridylamino)styrene]-N-ylmethylbenzothiazole iodonium salt is the same as in Example 2.
[0058] (4) Weigh the corresponding sodium tetrafluoroborate corresponding to 20 times the equivalent of the product in (3), heat and reflux in water and ethanol solution for 24 hours to obtain the compound shown in formula (IX).
[0059]
[0060] (5) 54 mg of the compound represented by the formula (VI) was reacted with 18.8 mg of copper nitrate in water to obtain the fluorescent chemic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com