Preparation and application of recombinant plectasin
A technology of plectasin and yeast, applied in the field of treatment or prevention of Gram-positive bacteria, especially streptococci, preparation of recombinant plectasiacin by yeast genetic engineering bacteria, the field of preparation of recombinant plectasiacin, Achieve good pH stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The construction of embodiment 1 plectasin genetically engineered bacteria
[0045] 1.1 Design of Plectasin gene based on yeast preferred codons
[0046] The Plectasin gene was artificially designed according to the codon usage preference of Pichia pastoris (http: / / www.kazusa.or.jp / codon / ), and the obtained sequence is shown in SEQ ID NO.1.
[0047] 1.2 Construction of recombinant plectasin expression vector
[0048] At the 5'-end of the Plectasin gene, a restriction endonuclease XhoI cleavage site, which is not available in the Plectasin gene but on the multiple cloning site of the vector, is designed, and the yeast Kex2 cleavage site after the XhoI cleavage site is retained (KR) coding sequence, in order to cleave the signal peptide after secretion and expression, to obtain recombinant Plectasin, design the TAATAA terminator sequence and XbaI restriction site at the 3'-end of the gene, in order to terminate the expression of the polypeptide and construct the vector d...
Embodiment 2
[0055] Induced expression of embodiment 2 recombinant plectasin
[0056] 2.1 Induced expression at shake flask level
[0057] The recombinant Pichia pastoris with high expression level obtained by screening was cultured in BMGY medium with glycerol as carbon source at 29°C with shaking at 250rpm to OD 600 2-6 hours; centrifuge at 5000rpm for 5min, collect the cells, and resuspend the cells with methanol-containing BMMY medium to OD 600 1.0, 250rpm at 29°C for induction culture; methanol was added every 24h to a final concentration of 0.5%, and the fermentation broth was centrifuged at 120h of induction, and the supernatant was collected and freeze-dried for later use.
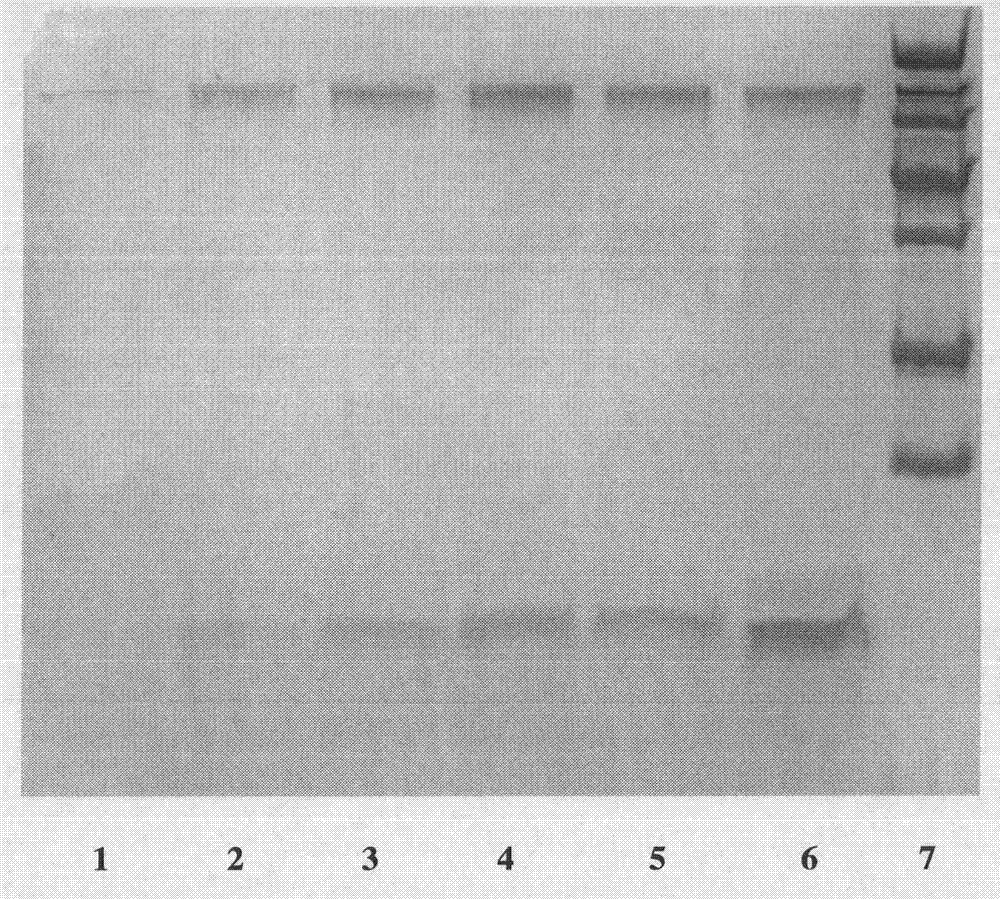
[0058] Such as figure 1 As shown, after induced expression, Tricine-SDS-PAGE electrophoresis showed that the secreted protein contained a 4.4kDa peptide.
[0059] 2.2 Induced expression at the level of 5L fermenter
[0060] Sartorius Bplus 5L fermenter (Sartorius, Germany) was used for high-density fermenta...
Embodiment 3
[0063] The purification of embodiment 3 recombinant plectasin
[0064] 3.1 Gel Filtration Chromatographic Separation of Recombinant Plectasin
[0065] The chromatographic column material is dextran gel Sephadex G-25 (purchased from Huamei Bioengineering Company), the separation range is 1000-5000Da, the purification conditions: the column bed volume is 40mL, the diameter-to-height ratio is 1:10, and deionized water is used for elution , the flow rate is 0.5mL / min, the detection wavelength: 280nm, after purification, it is tested by antibacterial test, and the components with the highest antibacterial activity are collected for further reversed-phase high performance liquid chromatography.
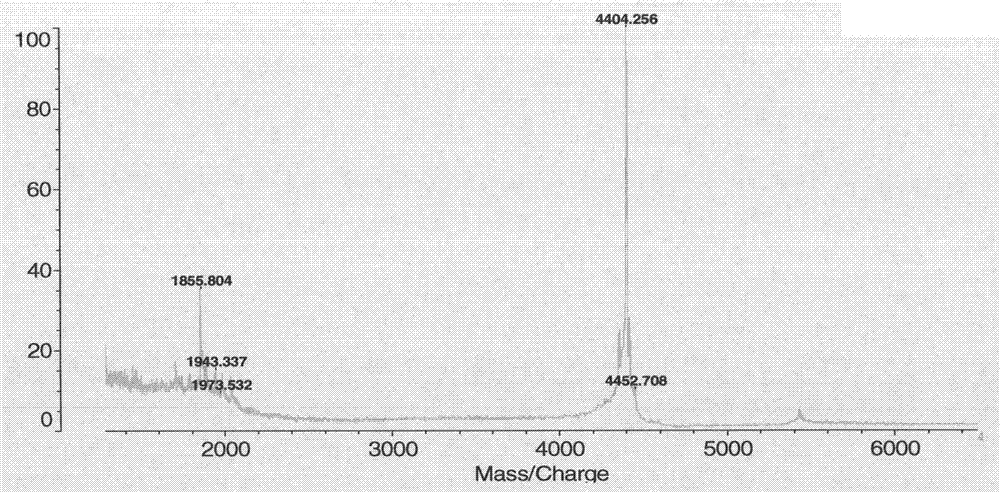
[0066] 3.2 Reversed-phase high-performance liquid chromatography of recombinant plectasin
[0067] Columns are XBridge TM BEH300 C18 (5μm, 4.6mm×250mm), using TFA-containing water and acetonitrile as the mobile phase, separated by gradient elution mode, dissolved the sample in ultrapure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com