Formula, preparation method and application of omeprazole sodium liposome composite medicament
A technology of omeprazole sodium lipid and liposome, which can be used in pharmaceutical formulation, liposome delivery, drug combination and other directions, can solve the problems of batch fluctuation, energy consumption and time-consuming, increasing leakage rate, etc., and achieve zero waste and three wastes. Emissions, plant saving, and the effect of improving the therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
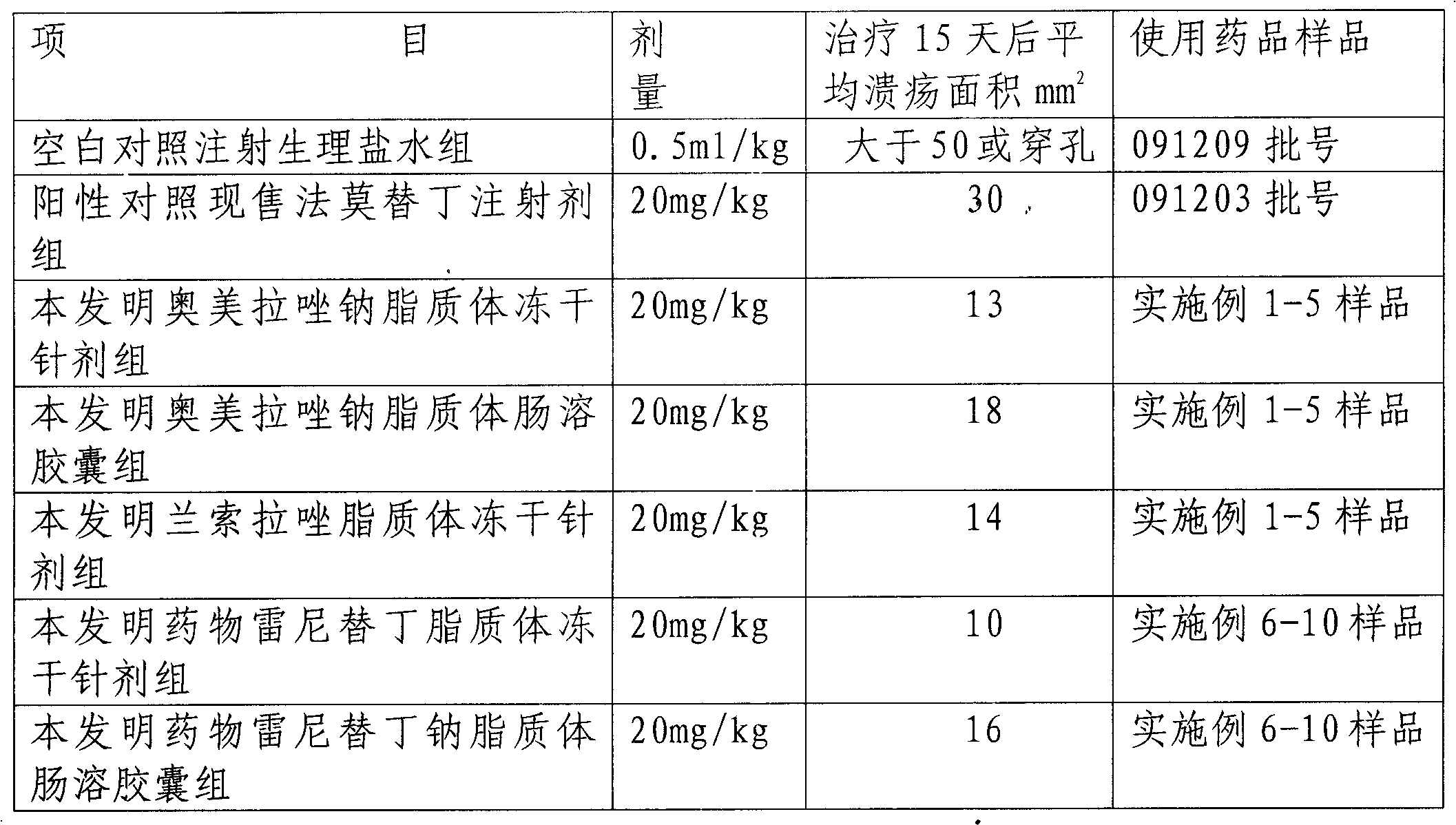
Examples
Embodiment 1
[0057] The raw material drug is a highly water-soluble liposome combination drug. The molar ratio of each effective raw material component of the standard is as follows:
[0058] 1. The raw material is omeprazole sodium 0.05
[0059] 2. Phospholipid raw materials are soybean lecithin and polyene phosphatidylcholine
[0060] Mole ratio 1:0.1 composition 0.50
[0061] 3. The antioxidant is reduced glutathione 0.01
[0062] 4. The molecular diluent of phospholipid membrane is dimercaprol 1.00
[0063] 5. The dispersant and excipient of liposome drug-carrying body is xylitol 2.00
[0064] 6. Surfactant is sodium dehydrocholate 0.01
[0065] 7. Ethanol 80% (v / v) (when dry, evaporate to the fullest) appropriate amount
[0066] 8. Phosphate buffer solution for injection 0.05M pH value 5.0-8.0 appropriate amount
[0067] 9. Equal volumes of water for injection (when dry, evaporate to the limit) and phosphate buffer for injection
[0068] The preparation method is as described abo...
Embodiment 2
[0070] The raw material drug is a highly water-soluble liposome combination drug. The molar ratio of each effective raw material component of the standard is as follows:
[0071] 1. The raw material is omeprazole sodium 0.20
[0072] 2. Phospholipid raw materials are soybean lecithin and polyene phosphatidylcholine
[0073] Mole ratio 1:0.1 composition 4.00
[0074] 3. The antioxidant is reduced glutathione 0.06
[0075] 4. The molecular diluent of phospholipid membrane is dimercaprol 6.00
[0076] 5. The dispersant and excipient of liposome drug-carrying body is xylitol 8.00
[0077] 6. Surfactant is sodium dehydrocholate 0.05
[0078] 7. Ethanol 80% (v / v) (when dry, evaporate to the fullest) appropriate amount
[0079] 8. Phosphate buffer solution for injection 0.05M pH value 5.0-8.0 appropriate amount
[0080] 9. Equal volumes of water for injection (when dry, evaporate to the limit) and phosphate buffer for injection
[0081] The preparation steps and methods are th...
Embodiment 3
[0083] The raw material drug is a highly water-soluble liposome combination drug. The molar ratio of each effective raw material component of the standard is as follows:
[0084] 1. The raw material is omeprazole sodium 0.15
[0085] 2. Phospholipid raw materials are soybean lecithin and polyene phosphatidylcholine
[0086] Mole ratio 1:0.1 composition 3.06
[0087] 3. The antioxidant is reduced glutathione 0.05
[0088] 4. The molecular diluent of phospholipid membrane is dimercaprol 5.60
[0089] 5. The dispersant and excipient of liposome drug-carrying body is xylitol 7.70
[0090] 6. Surfactant is sodium dehydrocholate 0.04
[0091] 7. Ethanol 80% (v / v) (when dry, evaporate to the fullest) appropriate amount
[0092] 8. Phosphate buffer solution for injection 0.05M pH value 5.0-8.0 appropriate amount
[0093] 9. Equal volumes of water for injection (when dry, evaporate to the limit) and phosphate buffer for injection
[0094] The preparation steps and methods are th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com