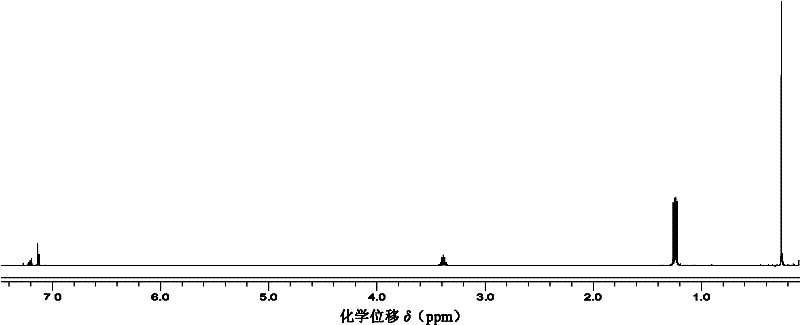
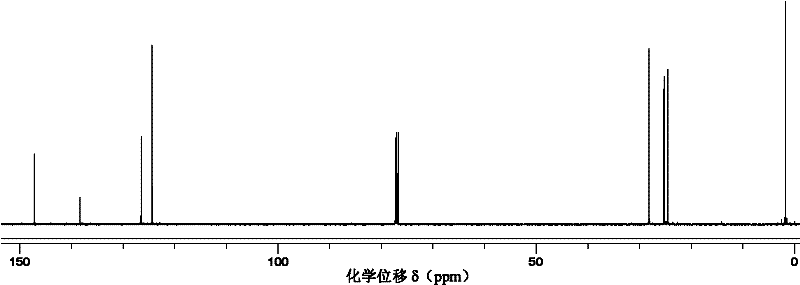
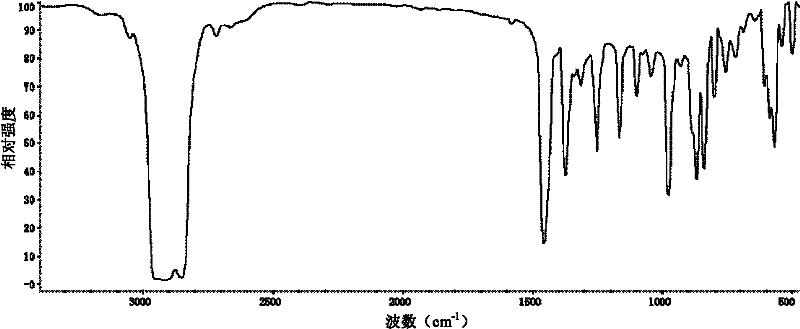
Method for preparing trisilicon thiol
A technology of silicon trithiol and trithiol, which is applied in the field of compound and its preparation, can solve the problems of high synthesis requirements, great influence of temperature, and complicated source of raw materials, and achieve the effect of mild reaction conditions and convenient product purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Preparation of trimethylsilyl-(2,6-diisopropylphenyl) aminosilyl trichloride
[0036] (a). Choose a 1L Schlenk round-bottom flask, and after sufficient nitrogen replacement, add 2,6-diisopropylaniline (0.20mol, 37.9mL) and 200mL ether solvent, start stirring, and use isopropyl The mixture of alcohol and liquid nitrogen was cooled to -78°C, n-butyllithium (0.20mol, 2.5M n-heptane solution 80.0mL) was slowly added dropwise, and the mixture was naturally warmed to room temperature for 6h.
[0037] (b). Cool the resulting reaction solution again to -78°C under constant stirring, and slowly add trimethylchlorosilane (0.20mol, 25.8mL) dropwise, and the dropwise addition is completed within 30 minutes. After the addition is complete, it is naturally raised to room temperature and stirred. After that, the precipitate was filtered off under a nitrogen atmosphere. Under constant stirring at room temperature, n-butyl lithium (0.20 mol, 2.5M n-heptane solution 80.0 mL) was slowly ...
Embodiment 2
[0054] (1) Preparation of triethylsilyl-(phenyl)aminosilyl trichloride
[0055] (a). Choose a 1L Schlenk round bottom flask, after full nitrogen replacement, add aniline (0.20mol, 18.2mL) and 200mL anhydrous toluene solvent in turn, start stirring, and use a mixture of isopropanol and liquid nitrogen After cooling to -30°C, methyl lithium (0.20 mol, 125 mL of 1.6M ether solution) was slowly added dropwise, and the reaction was allowed to rise to room temperature for 6 hours.
[0056] (b). Cool the resulting reaction solution to -30°C again under constant stirring, and slowly add triethylchlorosilane (0.20mol, 33.3 mL) dropwise. After the addition is complete, it is naturally raised to room temperature and stirred. After that, the precipitate was filtered off under a nitrogen atmosphere. Under constant stirring at room temperature, n-butyl lithium (0.20 mol, 2.5M n-heptane solution 80.0 mL) was slowly added dropwise to the filtrate. After the gas evolution stopped, stirring was c...
Embodiment 3
[0064] (1) Preparation of trimethylstannyl-(2,6-dimethylphenyl)amino silicon tribromide
[0065] (a). Choose a 500mL Schlenk round-bottomed flask, after full nitrogen replacement, add 2,6-dimethylaniline (0.10mol, 12.3mL) and 150mL ether solvent, start stirring, and cool the solution to -50 At °C, ethylmagnesium bromide (0.10mol, 33.5mL of a 3.0M ether solution) was slowly added dropwise, and the reaction was allowed to rise to room temperature for 12h.
[0066] (b). Cool the obtained reaction solution again to -50°C under constant stirring, slowly add trimethyltin chloride (0.10mol, 20.2mL) dropwise, and naturally rise to room temperature. After stirring for about 12 hours, without filtering the precipitate, n-butyl lithium (0.10 mol, 2.5M n-heptane solution 40.0 mL) was directly added dropwise to the filtrate at room temperature. After the generated gas ceased to escape, it was stirred for 12 hours under a nitrogen atmosphere.
[0067] (c). The obtained solution was further coole...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com