Method for preparing hydrophilic drug microsphere through spray drying process
A hydrophilic drug, spray-drying technology, applied in pharmaceutical formulations, organic active ingredients, medical preparations with non-active ingredients, etc. It is difficult to mass-produce and other problems, and achieves the effect of overcoming a large volume of oily substances and a large amount of volatile solvents for washing, rounding the shape, and reducing organic residues.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
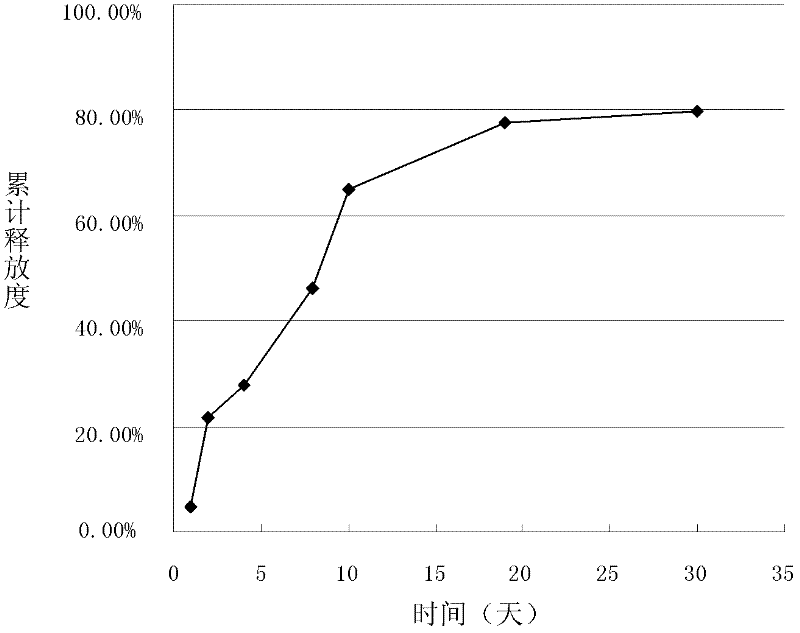
[0039]The preparation of embodiment 1 doxorubicin hydrochloride sustained-release microspheres
[0040] Raw material prescription:
[0041]
[0042]
[0043] The preparation process is as follows:
[0044] (1) Configure spray liquid
[0045] Dissolve the acrylic resin II in the ethanol solution, then add glyceryl monostearate and castor oil and stir evenly;
[0046] (2) Primary package of raw materials
[0047] Uniformly disperse doxorubicin hydrochloride in sesame oil containing Span80 to prepare S / O 1 type suspension.
[0048] (3) Homogeneous emulsification of spray liquid
[0049] Add the suspension prepared in (2) to the spray liquid obtained in (1) for homogeneous emulsification, the homogenization temperature is 50 °C, the speed of the high-speed shear homogenizer is 14000 rpm, and the time is 6 minutes to obtain S / o 1 / O 2 type spray emulsion;
[0050] (4) Spray drying: Spray dry the above-mentioned spray emulsion, the air inlet temperature is 160°C, the...
Embodiment 2
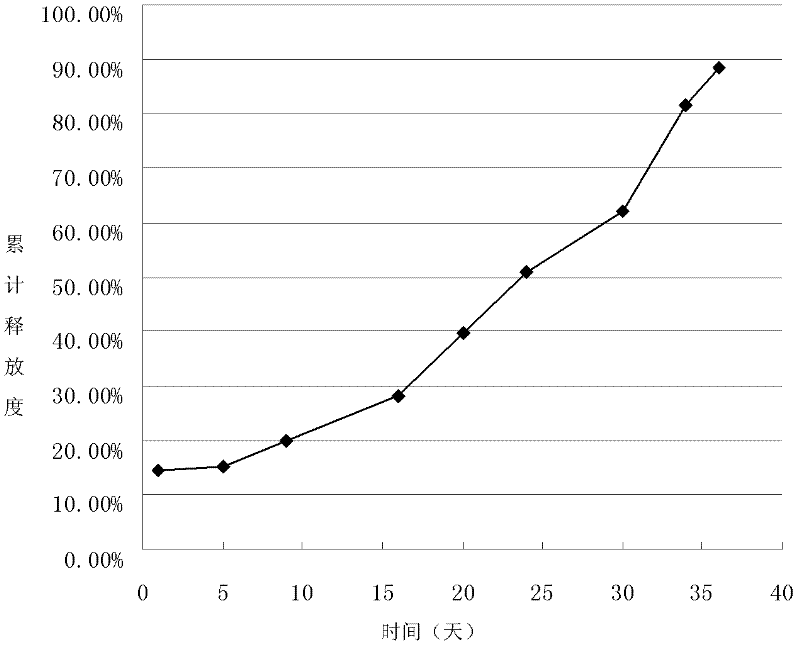
[0052] Example 2 Preparation of 5-fluorouracil sustained-release microspheres
[0053] Raw material prescription:
[0054]
[0055] The preparation process is as follows:
[0056] (1) Configure spray liquid
[0057] Dissolve acrylic resin Eudragit L100 and acrylic resin Eudragit S100 in ethanol solution, then add glyceryl monostearate and castor oil and stir well.
[0058] (2) Primary package of raw materials
[0059] Disperse 5-fluorouracil evenly in peanut oil containing Span80 to make S / O 1 type suspension.
[0060] (3) Homogeneous emulsification of spray liquid
[0061] Add the suspension prepared in (2) to the spray liquid obtained in (1) for homogeneous emulsification, the homogenization temperature is 50°C, the speed of the high-speed shear homogenizer is 10000 rpm, and the time is 9min, to obtain S / o 1 / O 2 type spray emulsion.
[0062] (4) Spray drying: Spray dry the above-mentioned spray emulsion, the air inlet temperature is 145°C, the air outlet temper...
Embodiment 3
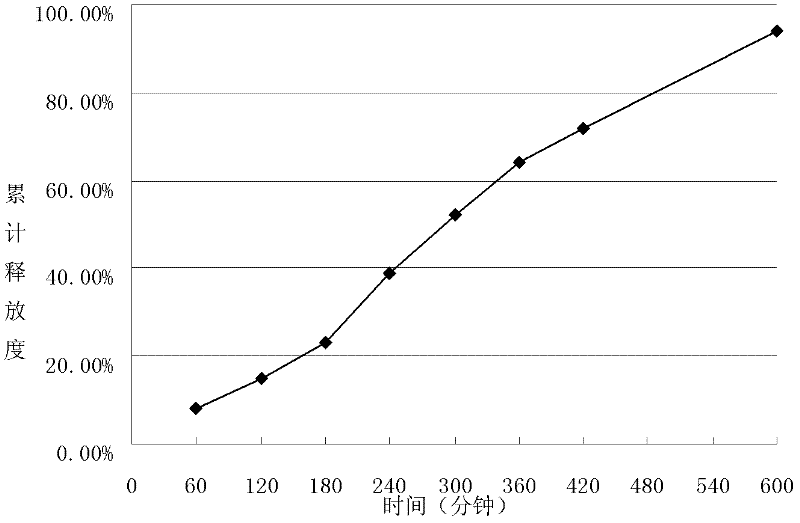
[0064] The preparation of embodiment 3 metformin hydrochloride sustained-release microspheres
[0065] Raw material prescription:
[0066]
[0067] The preparation process is as follows:
[0068] (1) Configure spray liquid
[0069] Dissolve acrylic resin Eudragit L100 in ethanol solution, then add glyceryl monostearate and castor oil and stir well.
[0070] (2) Primary package of raw materials
[0071] Disperse metformin hydrochloride evenly in sesame oil containing Span80, and prepare S / O 1 type suspension.
[0072] (3) Homogeneous emulsification of spray liquid
[0073] Add the suspension prepared in (2) to the spray liquid obtained in (1) for homogeneous emulsification, the homogenization temperature is 50°C, the speed of the high-speed shear homogenizer is 20000 rpm, and the time is 4min, to obtain S / o 1 / O 2 type spray emulsion.
[0074] (4) spray drying
[0075] The above-mentioned spray emulsion was spray-dried, the inlet air temperature was 155°C, the out...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com