Method for purifying recombinant aspergillus flavus uricase expressed by bacillus coli
A technology of uric acid oxidase and Escherichia coli, applied in the field of protein separation and purification, can solve the problems of increasing purification cost and production cycle, expensive protease price, long enzyme digestion time, etc., and achieves easy automation, low cost and short production cycle. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
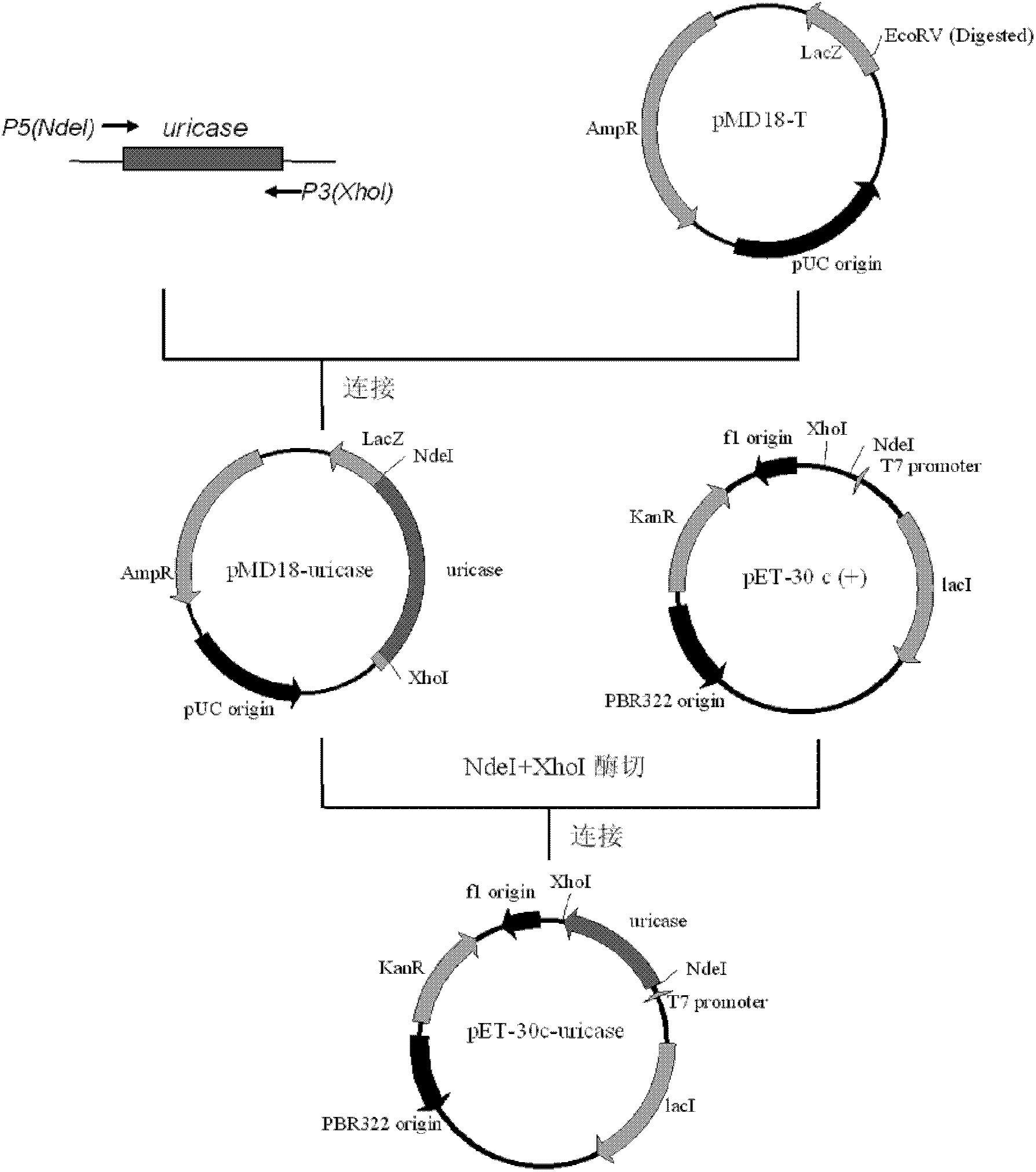
[0057] A method for isolating and purifying recombinant Aspergillus flavus uricase expressed by Escherichia coli, the steps are:
[0058] A. Cloning of Aspergillus flavus urate oxidase cDNA:
[0059] Aspergillus flavus was cultivated for 3 days, the bacterial liquid was filtered, and the mycelium was collected. The total RNA of Aspergillus flavus mycelium was extracted according to the instructions of the Invitrogen Trizol RNA kit, and the total RNA of Aspergillus flavus mycelium was extracted with UricaseP1 (5-GGAATTCCATATGTCCGCAGTAAAAGCAGCCCGCTACG-3) and UricaseP2 (5-AGCCTCGAGTTATTACAATTTAGACTTCAGAGAGGACCG- 3) As primers, perform RT-PCR amplification according to the operating instructions of the RT-PCR kit of Promega Company. The amplified product (about 900bp) was connected to the T cloning vector pMD18-T of Takara Company, and Escherichia coli DH5α (purchased from Sangon Biotech (Shanghai) Co., Ltd.) was transformed by electroporation, and white colonies were picked for i...
Embodiment 2
[0079] In vitro biological activity assay of recombinant Aspergillus flavus uric acid oxidase:
[0080] Add 3 mL of 100 μmol / L uric acid solution dissolved in 3 mL of TEA buffer solution (7.5 g / L triethanolamine, 0.38 g / L EDTA, pH 8.9) into a 4 mL cuvette, then add 1 μg of purified urate oxidase, at 25 °C Add 0.5 mL of 20% (mass volume ratio) KOH to terminate the reaction after reacting for 5 min under the condition. The activity of the enzyme is obtained by detecting the decrease of the concentration of uric acid reflected by the decrease of the absorbance value at 292nm. Enzyme activity definition: under the conditions of pH 8.9 and 30°C, the amount of enzyme required to catalyze the oxidation of 1 μmol of uric acid per minute is one unit. Finally, the specific activity of the purified Aspergillus flavus uricase was measured to be 30 IU / mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com