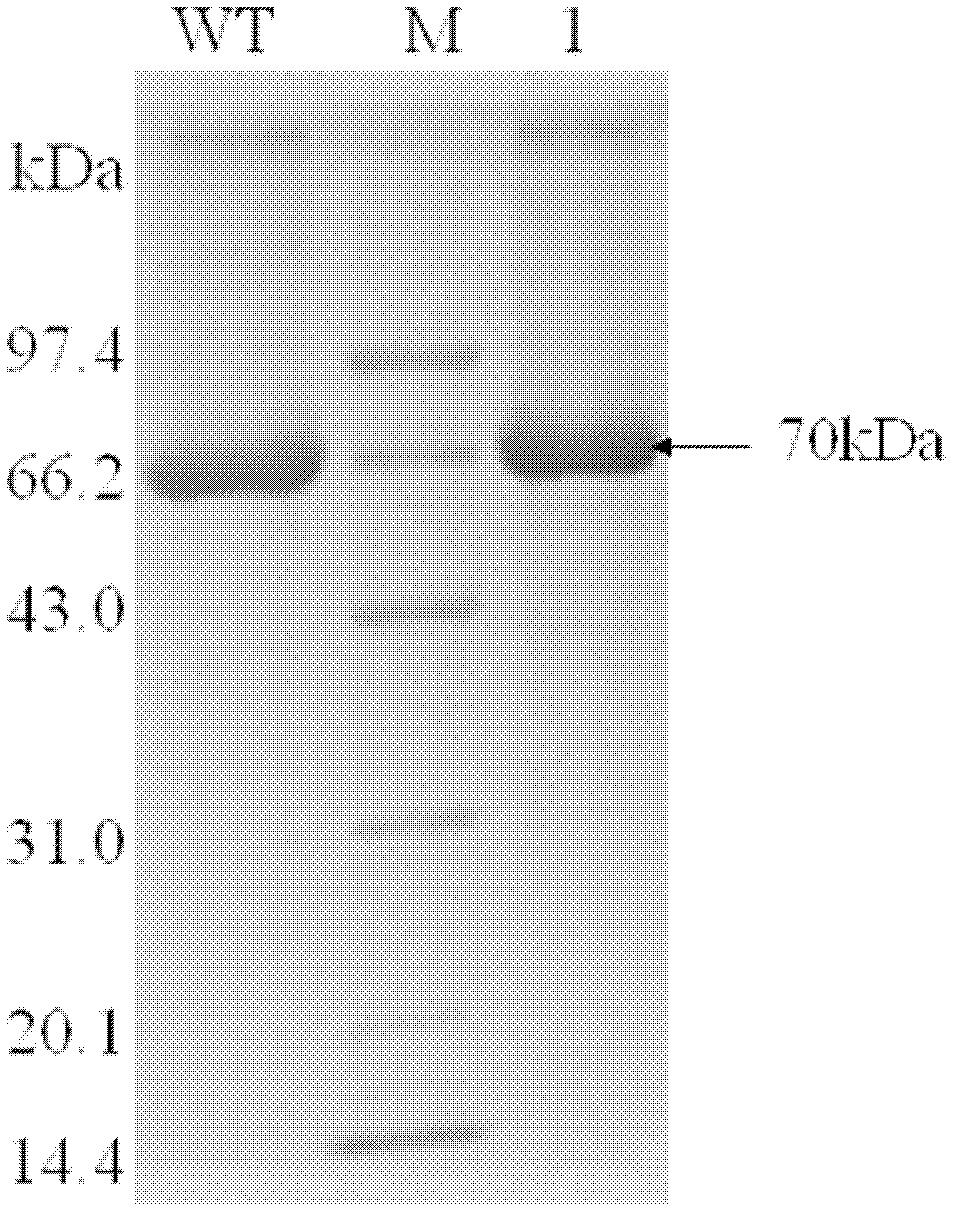Construction of heat-resistant beta-galactosidase mutant
A technology of galactosidase and mutants, which is applied in the field of protein engineering, can solve the problems of not being able to cooperate with pasteurization, milk flavor and color changes, etc., and achieve the effects of improving flavor and taste, saving sucrose consumption, and increasing sweetness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
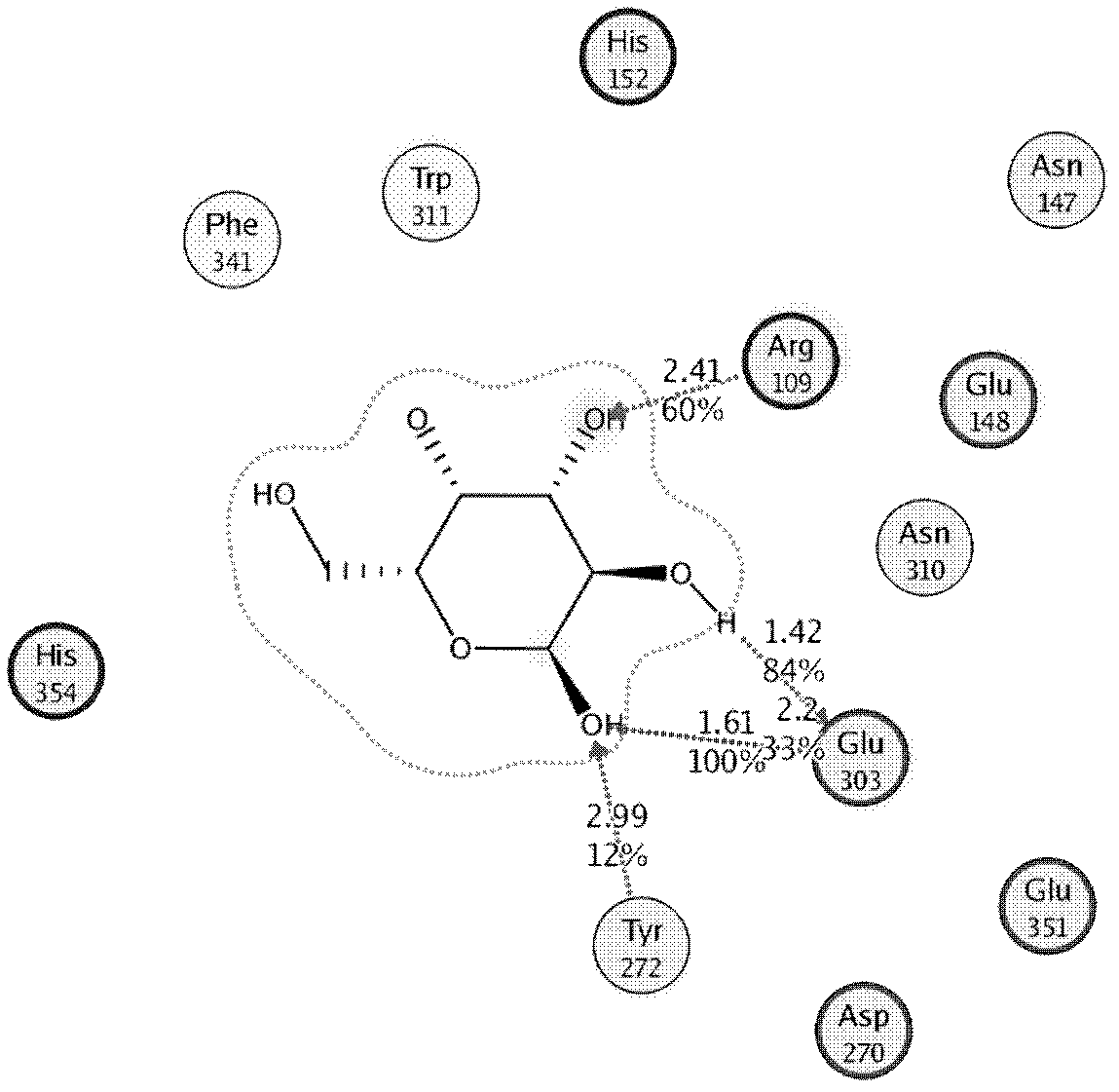
[0034] Identification of remodeling sites for thermostable β-galactosidase BgaB.
[0035] (1) Test method:
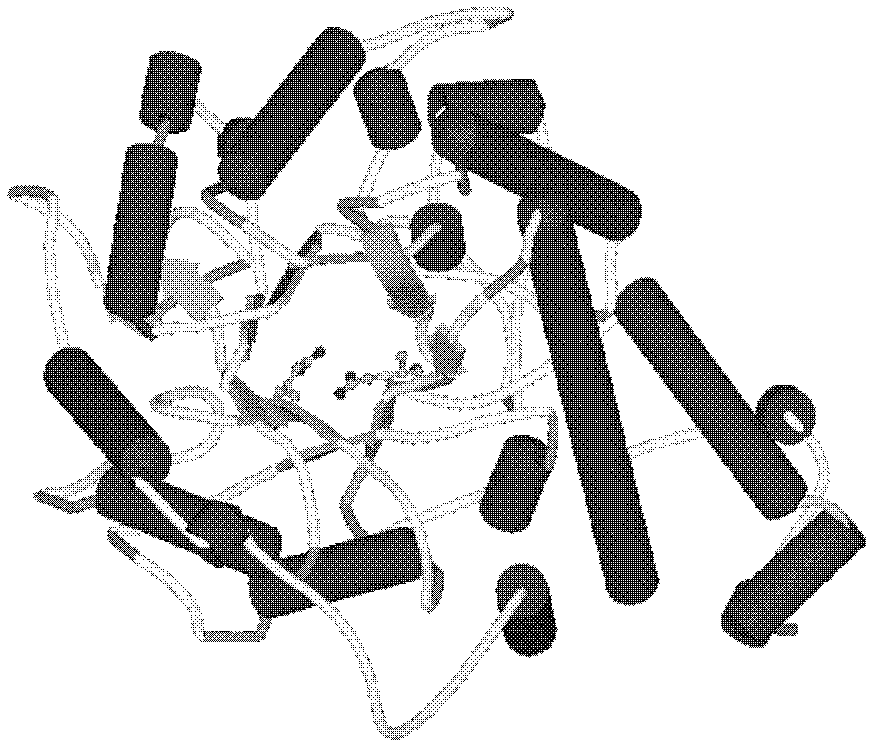
[0036] Construction of BgaB molecular model and substrate complex model
[0037] All calculations were completed by the CDOCKER calculation program provided by Accelerys DiscoveryStudio 2.1 software (Accelerys Software Inc., Accelrys Discovery Studio 2.1, San Diego, 2008; G.Wu, D.H.Robertson, C.L.Brooks, M.Vieth, Detailed analysis of grid-based molecular docking: a case study of CDOCKER-A CHARMm-based MD docking algorithm, J. Comp. Chem, 24(2003) 1549-1562).
[0038] Determination method of Michaelis constant Km and inhibition constant Ki
[0039] The concentration of ONPG is prepared from 0.8-10mmol / L, and different concentrations of lactose, galactose and glucose are added to the substrate analogue inhibition experiment. The reaction system is the same as the enzyme activity test, and the enzyme solution purified by a nickel column is added, and the difference is me...
Embodiment 2
[0052] Construction of Thermostable β-Galactosidase BgaB Mutant
[0053] 1. Site-directed modification of the active site of the heat-resistant β-galactosidase gene bgab gene
[0054] The plasmid pKK223-3-bgab (Dong Y. N., Liu X.M., Chen H.Q., et al..Enhancement of the hydrolysis activity of beta-galactosidase from Geobacillus stearothermophilus by saturation mutagenesis.Journal of Dairy Science, 2011, 94(3):1176-1184) was used as a template, and a pair of primers (Sequence 1 and Sequence 2) were used to carry out site-specific modification of the bgab gene by the method of full plasmid amplification , the PCR program is: 95°C for 30s, 55°C for 30s, 68°C for 6.6min, 16 cycles. PCR reaction system: 5 μl dNTPs (2mM), 5 μl 10×Buf., 1 μl KOD plus (high-fidelity polymerase, Toyobo), 2 μl MgSO 4 (25mM), 1.5 μl each of upstream and downstream primers, and 1 μl template.
[0055] 2. Screening of Clones
[0056] The amplified PCR product was digested with endonuclease Dpn I (Fermen...
Embodiment 3
[0058] Inoculate the transformant of the mutant enzyme in 250mL LBA liquid medium and culture at 200r / min at 37°C until OD 600When it reaches 0.6~0.8, add IPTG (isopropyl-β-D-thiogalactoside, final concentration 1mM), induce for 20h, collect the bacteria by centrifugation, resuspend with 50mM phosphate buffer (pH 6.5), and place in ice bath Ultrasound was used to break the wall, centrifuged at 10000r / min for 30min, and water-bathed at 60°C for 30min to remove some heat-labile foreign proteins, and the supernatant was purified by Ni-NTA agarose column (see Example 3 for details);
[0059] 4. Dialysis, freeze drying
[0060] The eluate was collected, and purified enzyme was obtained after dialysis and freeze-drying.
[0061] Example 3
[0062] Expression and purification of mutant BgaB-F341T of thermostable β-galactosidase BgaB
[0063] 1. Experimental method: The recombinant mutant enzyme contains 6 histidine tags at the C-terminus. After prokaryotic expression, the purified...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com