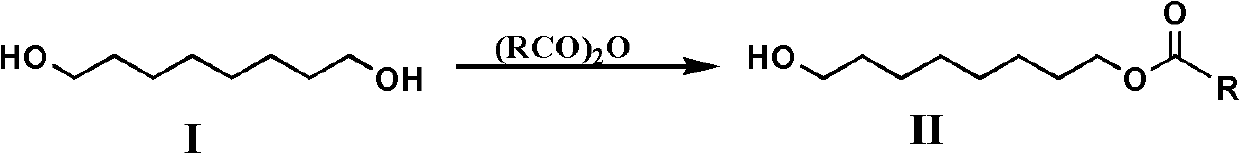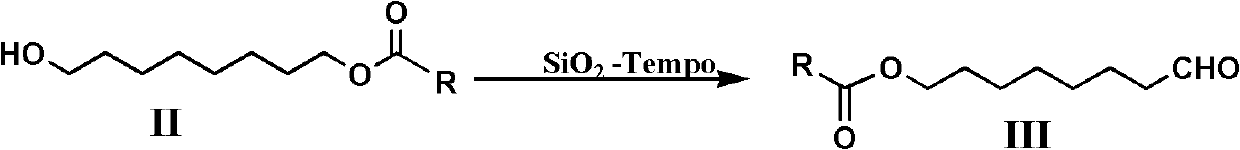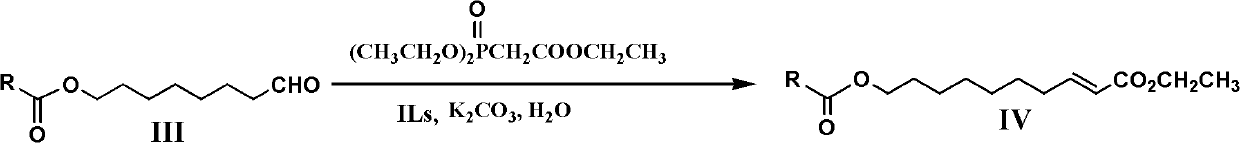The preparation method of royal jelly acid
A technology of royal jelly acid and acid anhydride, which is applied in the field of preparation of royal jelly acid, can solve the problems of unstable Grignard reagent, high requirements for reaction conditions, and many by-products, and solve the problems of non-recyclable use, high price, and high selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] SiO 2 - Preparation of Tempo:
[0043] Add 30g of Tempo, 80mL of dimethylformamide and 70g of nano-silica into a 250ml round bottom flask, heat and stir at 90°C for 1 hour, and remove the solvent to obtain the supported product SiO 2 -Tempo 100g, dry for later use.
Embodiment 1
[0045] Add 5 grams (0.034mol) of 1,8-octanediol, 40mL of dry tetrahydrofuran and 5.47mL (0.068mol) of pyridine in a 100mL single-necked bottle, and add 3.18mL (0.034mol) of acetic anhydride dropwise to the mixture under stirring in an ice bath, After 30 minutes of dripping, the reaction was continued for 4.5 hours. This reaction adopts TLC to detect the degree of progress of the reaction (developing agent is sherwood oil / ethyl acetate (volume ratio 1: 1), R f = 0.5). After the reaction is complete, remove tetrahydrofuran and pyridine under vacuum, dissolve the residue with dichloromethane, wash with water, separate the organic phase, anhydrous MgSO 4 Dry, filter, and put the filtrate at about 0°C to crystallize overnight. Unreacted 1,8-octanediol (1 gram) was removed by filtration and reclaimed, and the solvent was evaporated from the filtrate to obtain 4.26 grams of a light yellow oily liquid product of the general formula II compound (R is methyl, i.e. 8-acetoxyoctanol). ...
Embodiment 2
[0054] Add 10 grams (0.068mol) of 1,8-octanediol, 70mL of dry tetrahydrofuran and 10.3mL (0.128mol) of pyridine in a 250mL single-necked bottle, and add 6mL (0.064mol) of acetic anhydride dropwise to the mixture under stirring in an ice bath, 40 Minutes dripping, dripping and continue to react for 4h. After the reaction was complete, after removal of tetrahydrofuran and pyridine in vacuo, the residue was washed with CH 2 Cl 2 Dissolve, wash with water, separate organic layer, anhydrous MgSO 4 Dry, filter, and put the filtrate at about 0°C to crystallize overnight. Unreacted 1,8-octanediol (2 grams) was removed by filtration and reclaimed, and the filtrate was evaporated to obtain 11.8 grams of a light yellow oily liquid product general formula II compound (R is methyl, i.e. 8-acetoxy octanol) , the yield was 98.0% (excluding the recovered 1,8-octanediol).
[0055] Add 11.8 grams (0.063mol) of 8-acetoxy octanol and 80mL of methylene chloride in a 250mL three-neck round bott...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com