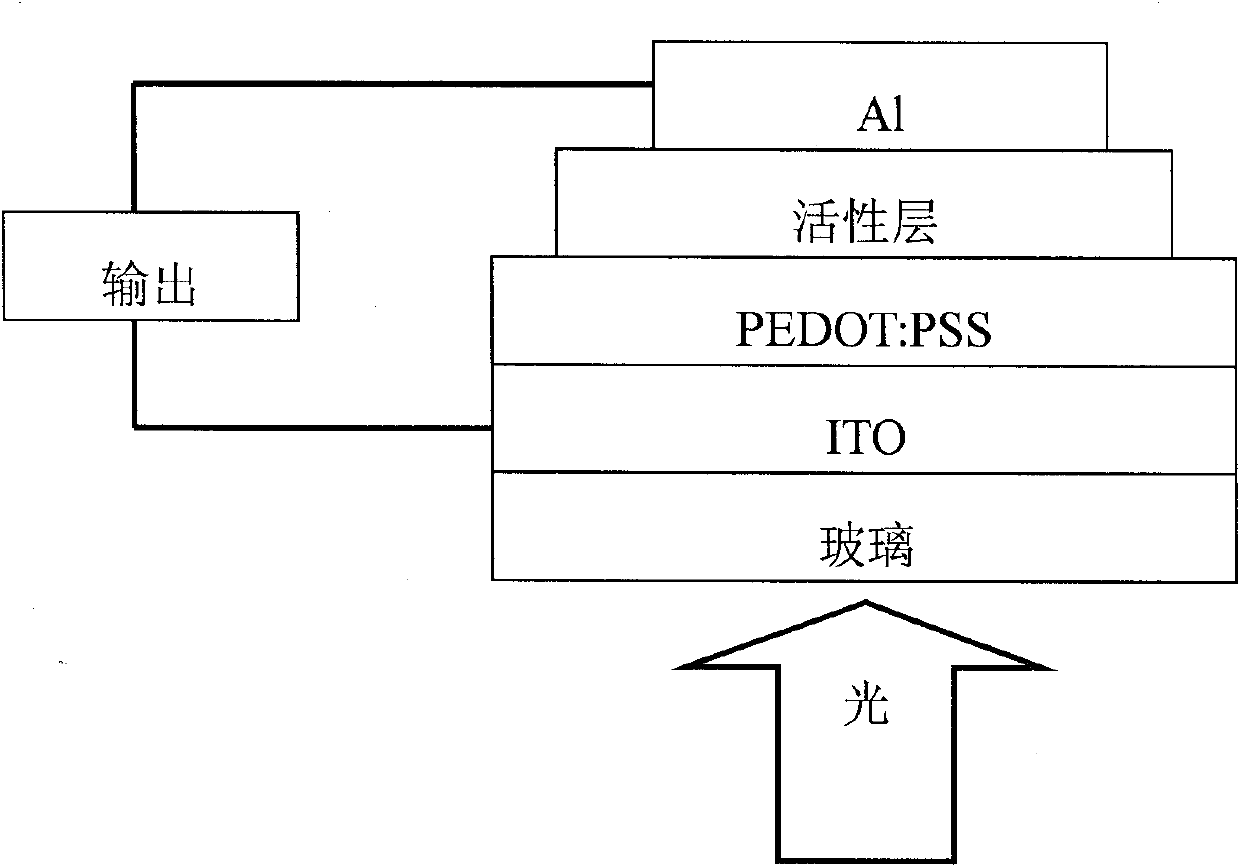
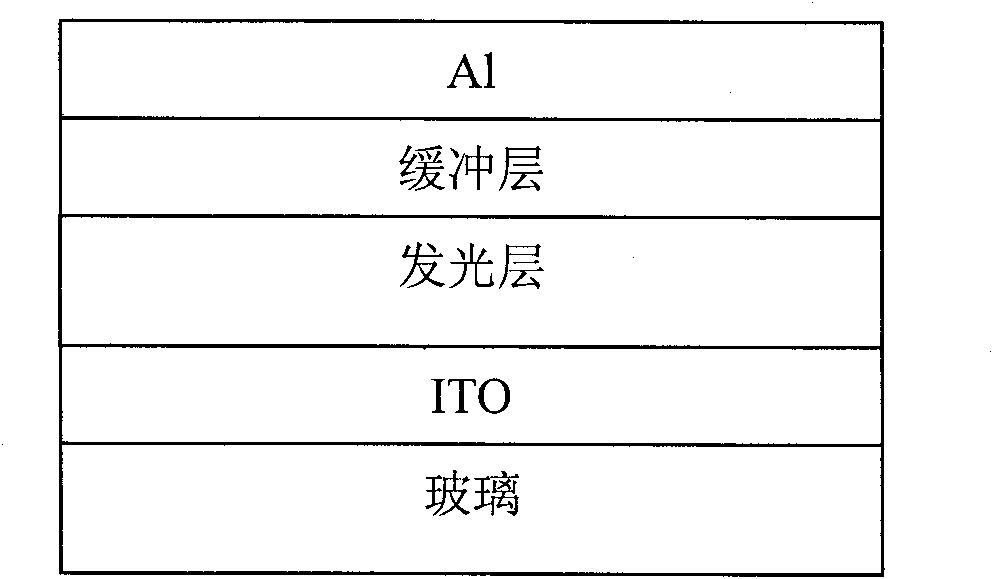
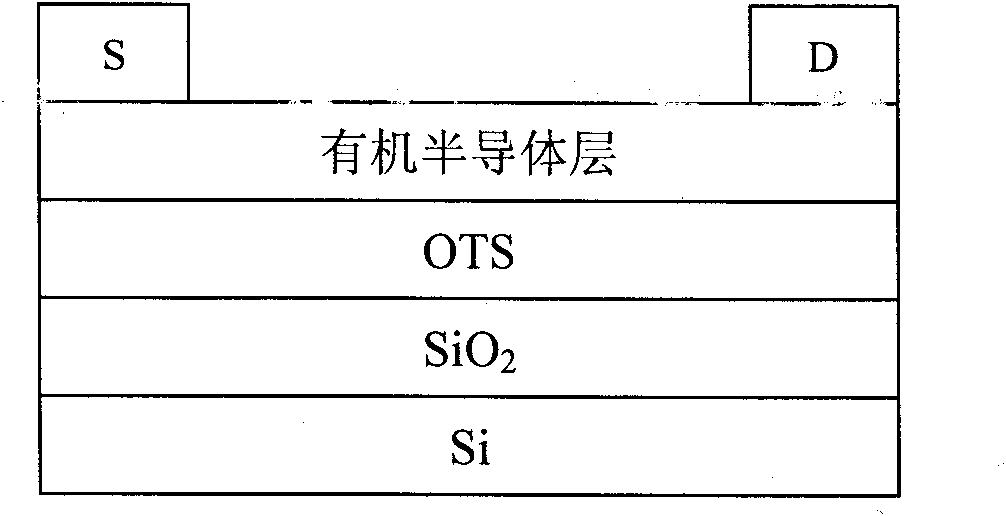
Perylenetetracarboxylic acid diimide copolymer containing bisthienopyrrole unit and its preparation method and application
A technology of perylene tetracarboxylic acid diimide copolymer and perylene tetracarboxylic acid diimide, which is applied in the fields of electrical components, semiconductor/solid-state device manufacturing, and electric solid-state devices, etc., can solve the problem that the devices are prone to phase separation and dissolution. It can solve the problems of poor performance, poor film-forming processing performance, etc., to achieve the effects of excellent charge transport performance, wide absorption range and low equipment requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Poly N, N'-bis-(3,4,5-tri-methylbenzene)-3,4,9,10-perylenediimide-N-hexylbisthieno[3,2- b: 2′,3′-d]pyrrole:
[0044]
[0045] Under the protection of nitrogen, to the compound N,N'-bis-(3,4,5-tri-methylbenzene)-1,7-dibromo-3,4,9,10-perylene diimide 0.5 mmol, 2,6-bistrimethyltin-N-hexylbisthieno[3,2-b:2′,3′-d]pyrrole 0.5mmol in DMF (18mL) solution was bubbled for 0.5h to remove residual Oxygen; then add Pd 2 (dba) 3 (0.0.14g, 0.015mol) and P(o-Tol) 3 (0.0083g, 0.027mmol) and bubbling for 0.5h to remove residual oxygen, and then heated to 80 ° C for 48 hours; the reaction mixture was added dropwise to methanol for sedimentation treatment, then suction filtered, washed with methanol, and dried , to obtain the heteropolymer colloid; then dissolve it with toluene to obtain the toluene solution of the copolymer; then, add the toluene solution to the aqueous solution of sodium diethyldithiocarbamate, heat and stir the mixed solution at 90°C, and dissolve the m...
Embodiment 2
[0046] Example 2 Poly N, N'-bis-(3,4,5-tri-methoxybenzene)-3,4,9,10-perylenediimide-N-octylbisthieno[3, 2-b: 2',3'-d]pyrrole:
[0047]
[0048] Under the protection of nitrogen, to the compound N,N'-bis-(3,4,5-tri-methoxybenzene)-1,7-dibromo-3,4,9,10-perylene diimide 0.5mmol, 0.5mmol of 2,6-bistrimethyltin-N-octylbithieno[3,2-b:2′,3′-d]pyrrole in dioxane (15mL) solution was bubbled 0.5h to remove residual oxygen; then add Pd(PPh 3 ) 2 Cl 2 10mg, and bubbling for 0.5h to remove residual oxygen, and then heated to 85°C for 36 hours; the reaction mixture was added dropwise into methanol, settled, then suction filtered, washed with methanol, and dried to obtain a heteropolymer Colloid; then dissolved in toluene to obtain the toluene solution of the copolymer; then, add the toluene solution to the aqueous solution of sodium diethyldithiocarbamate, heat and stir the mixed solution at 90°C, and pass the mixed solution through the column of alumina Chromatography, isolate the...
Embodiment 3
[0049] Example 3 Poly N, N'-bis-(3,4,5-tri-octyloxybenzene)-3,4,9,10-perylene diimide-N-eicosylbisthieno[ 3,2-b:2′,3′-d]pyrrole:
[0050]
[0051] Under the protection of nitrogen, to the compound N, N'-bis-(3,4,5-tri-octyloxybenzene)-1,7-dibromo-3,4,9,10-perylene diimide Bubble 0.5mmol of 0.5mmol, 2,6-bistributyltin-N-eicosylbisthieno[3,2-b:2′,3′-d]pyrrole in toluene / THF (30mL) solution h to remove residual oxygen; then add Pd(PPh 3 ) 4 8mg and bubbling for 0.5h to remove residual oxygen, and then heated to 80°C for 72 hours; the reaction mixture was added dropwise to methanol for sedimentation treatment, then suction filtered, washed with methanol, and dried to obtain a heteropolymer colloid; then dissolved in toluene to obtain the toluene solution of the copolymer; then, add the toluene solution to the aqueous solution of sodium diethyldithiocarbamate, heat and stir the mixed solution at 80°C, and pass the mixed solution through the column layer of alumina The copol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com