A kind of method for preparing 7-aminocephalosporanic acid
A technology for amino cephalosporanic acid and amino acid, which is applied in the field of preparing 7-amino cephalosporanic acid, can solve the problems of many impurities, many residual solvents, poor stability, etc., and achieves the reduction of the total amount of COD, the reduction of environmental requirements, and the few reaction steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
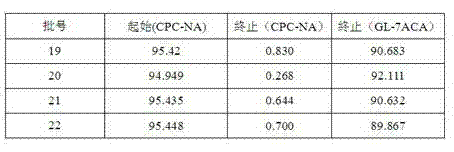
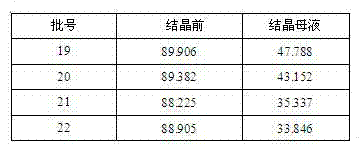
[0025] Add cephalosporin C sodium salt solution into the enzyme reactor, then add D-amino acid oxidase to oxidize, after hydrogen peroxide transfer, add GL-7 aminocephalosporanic acid acylase to cleavage, then crystallize, centrifugally filter, wash, Drying and testing yields 7-aminocephalosporanic acid with a purity of over 96%.
[0026] The concentration of the cephalosporin C sodium salt solution is 4%, and its preparation steps: take 48 grams of cephalosporin C sodium salt in a 1200ml beaker, add an appropriate amount of distilled water, after fully dissolving, adjust the pH to 7.0 with 3N ammonia water Left and right, remove the insoluble matter by vacuum filtration, add distilled water to 1200ml, and set aside in a constant temperature water bath at 20°C.
[0027] The input amount of the oxidase is 2500u oxidase per liter of cephalosporin C sodium salt solution.
[0028] The input amount of the acylase is 5000 u of the acylase per liter of cephalosporin C sodium salt so...
Embodiment 2
[0034] Add cephalosporin C sodium salt solution into the enzyme reactor, then add D-amino acid oxidase to oxidize, after hydrogen peroxide transfer, add GL-7 aminocephalosporanic acid acylase to cleavage, then crystallize, centrifugally filter, wash, Drying and testing yields 7-aminocephalosporanic acid with a purity of over 96%.
[0035] The concentration of the cephalosporin C sodium salt solution is 4%, and its preparation steps: take 48 grams of cephalosporin C sodium salt in a 1200ml beaker, add an appropriate amount of distilled water, after fully dissolving, adjust the pH to 7.0 with 3N ammonia water Left and right, remove the insoluble matter by vacuum filtration, add distilled water to 1200ml, and set aside in a constant temperature water bath at 20°C.
[0036] The input amount of the oxidase is 2500u oxidase per liter of cephalosporin C sodium salt solution.
[0037] The input amount of the acylase is 5000 u of the acylase per liter of cephalosporin C sodium salt so...
Embodiment 3
[0043] 7-Aminocephalosporanic acid double enzyme conversion HPLC analysis method
[0044] 1. Preparation of mobile phase
[0045] 1. Phase A
[0046] Weigh 1.542g of ammonium acetate and add 1000ml of distilled water to dissolve it, adjust the pH to 4.5 with glacial acetic acid, filter through a 0.45μm water filter and degas for 20 minutes.
[0047] 2. Phase B
[0048] Measure 900ml of phase A, add 100ml of acetonitrile and mix well, then degas for 20min.
[0049] 2. HPLC analysis conditions
[0050] Column type: Rp-8e, 5μm, LiChroCART 250-4
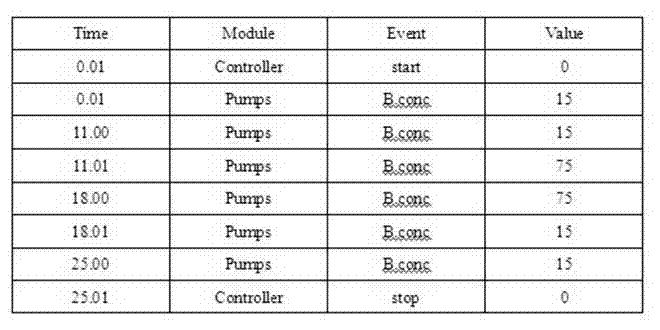
[0051] Total flow rate: 1.0ml / min
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com