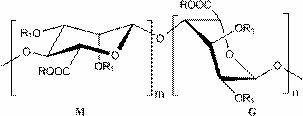
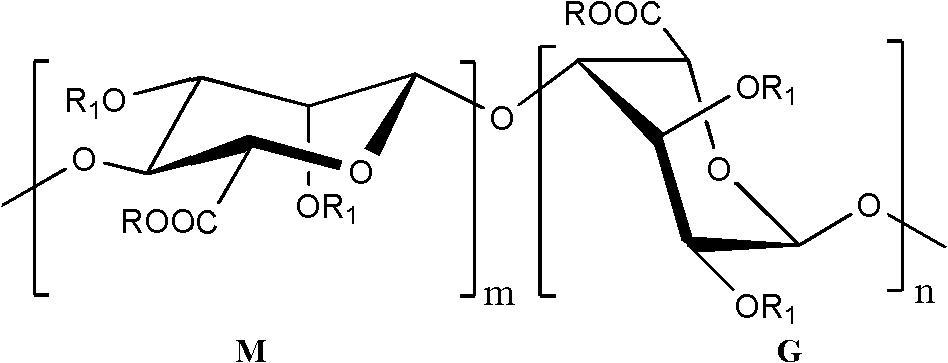
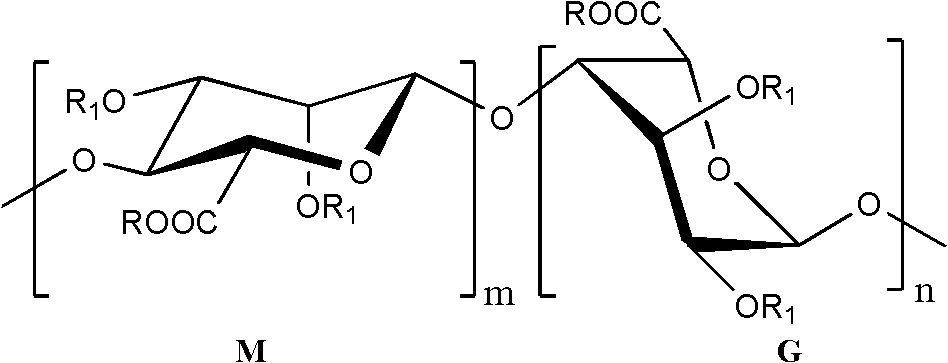
Low-molecular-weight polysaccharide sulfate and preparation method and application thereof
A technology of diester sodium alginate and low molecular weight, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., can solve problems such as adverse reactions and weakness, achieve improved solubility, reduce bleeding side effects, and prepare The method is simple and fast, and the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of Low Molecular Weight Sodium Alginate Diester
[0021] 9 g of sodium alginate diester (weight average molecular weight about 14kDa) was weighed and dissolved in 120 mL of distilled water to prepare a 7.5% sodium alginate diester solution. Add 0.1mol·L -1 FeSO 4 solution and 30% H 2 O 2 Aqueous solution, so that Fe in the reaction system 2+ and H 2 O 2 The concentrations reached the indicated final concentrations shown in Table 1. The reaction was sealed and shaken at 60 °C for 0.5 h. After the reaction was completed, follow 3 mg·mL -1 The amount of reaction system is added ascorbic acid to consume H 2 O 2 The reaction was terminated, rapidly cooled, 3 volumes of 95% ethanol were added, and the mixture was allowed to stand overnight at 4°C. Centrifuge at 3000r·min-1 for 20min to separate the precipitate and supernatant. Dissolve the precipitate in a small amount of water, pass through 732 cation exchange resin for desalting and purif...
Embodiment 2
[0029] Example 2: The main pharmacological activity experiment of low molecular weight sodium alginate diester
[0030] The main pharmacological activity test results of the low molecular weight sodium alginate diester prepared by the method of the present invention are as follows.
[0031] A: Anticoagulant activity test of low molecular weight sodium alginate diester
[0032] Insert a glass capillary tube into the posterior venous plexus of the mouse medial canthus to collect blood, and record the time from blood collection to the appearance of blood coagulation as an indicator for testing the coagulation time; after the tail of the mouse is decapitated, the time from the natural outflow of blood to the natural stop of blood is recorded. For testing the indicators of bleeding time. The above two indicators were used to detect and compare the anticoagulant effect and bleeding risk of low molecular weight sodium alginate diester with different molecular weights. The test resul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com