Hypolipidemic compounds, preparation method thereof and purpose thereof
A technology for reducing blood lipids and compounds, applied in the field of hypolipidemic compounds and their preparation, can solve problems such as minor applications, and achieve the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
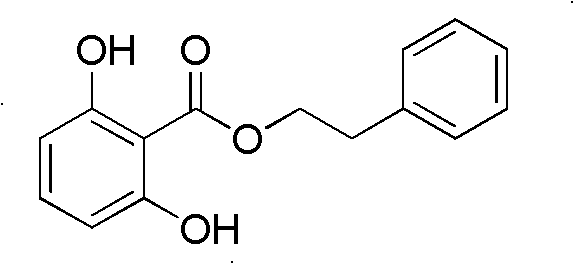
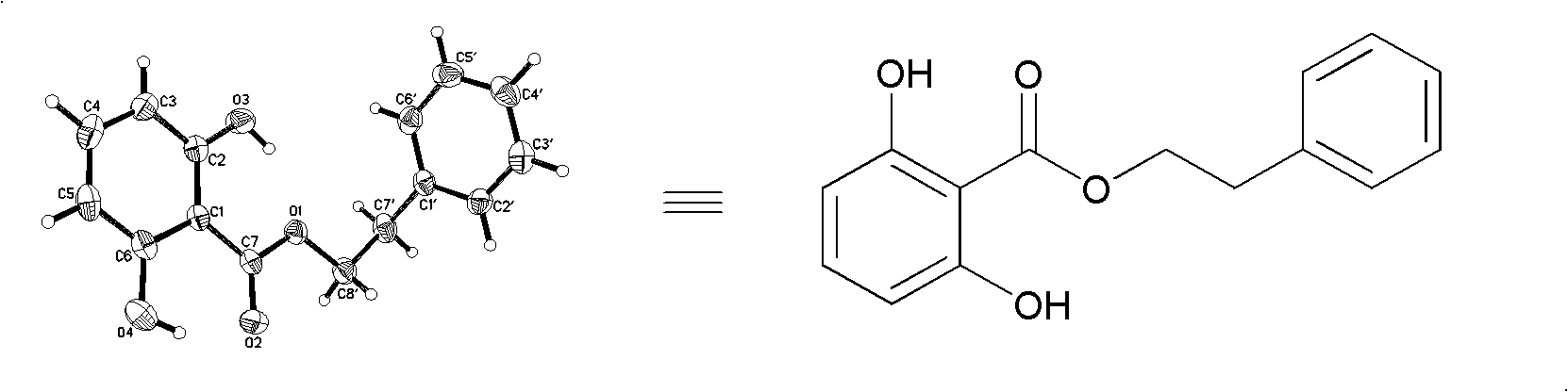
[0043] Example 1: Extraction scheme of 2,6-dihydroxybenzoic acid phenylethyl alcohol ester
[0044] 5Kg (dry weight) of the whole plant of Alderwort, crushed, extracted with 95% ethanol (50L×3), concentrated under reduced pressure to obtain 220g of total extract. The total extract was dissolved in 2.0L warm water (about 50°C), extracted with ethyl acetate (1.5L×3) after cooling, and concentrated under reduced pressure to obtain 80g of ethyl acetate. The ethyl acetate fraction was eluted by gradient silica gel column chromatography (CHCl3, CHCl3:CH3OH=50:1, 20:1, 10:1, 5:1, 2:1, CH3OH) and divided into 10 fractions (TLC for detection, The same parts are combined together); Fr2 is separated by silica gel column chromatography (petroleum ether: ethyl acetate=50:1) to obtain 4 small parts, wherein the second small part is obtained by silica gel column chromatography (petroleum ether: chloroform=3 : 1) 70 mg of the target compound was isolated, and spectroscopy and X-single crysta...
Embodiment 2
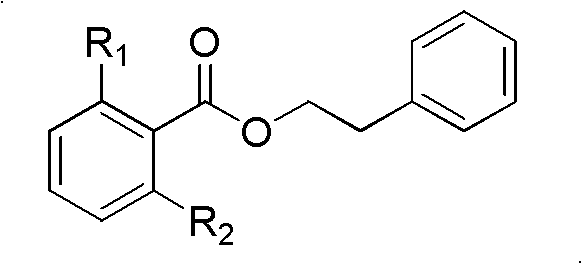
[0045] Embodiment 2: 2, the synthesis of phenylethyl alcohol ester (2) of 6-dimethoxybenzoate
[0046] Weigh 200mg of 2,6-dimethoxybenzoic acid, place it in a pre-dried round bottom flask, add 10mL of anhydrous CH2Cl2, stir; place the above device in an ice-water bath, and wait for the temperature of the mixture to drop to 0°C, add 250 mg of dicyclohexylcarbodiimide to the mixture, stir for 30 minutes, add 0.122 mL of phenethyl alcohol, 6 mg of 4-N, N-dimethyl-4-aminopyridine, stir at room temperature, TLC Detection, after the reaction is complete, filter directly, and concentrate the mother liquor to obtain a residue, which is separated by silica gel column chromatography (petroleum ether: chloroform = 2: 3) to obtain phenylethyl 2,6-dimethylbenzoate with a yield of 90.6%. Proton NMR spectrum (600MHz, CDCl 3 , δin ppm and TMS as internal standard): δ7.25~7.31(5H, m), 7.22(1H, m), 6.54(2H, d, J=8.2Hz), 4.54(2H, t, J=7.1Hz ), 3.78 (6H, s) and 3.06 (2H, t, J=7.1Hz). C NMR spec...
Embodiment 3
[0047] Embodiment 3: the synthesis of 2-hydroxyl-6-methoxyphenyl ethyl benzoate (3)
[0048] Place the 2,6-dimethylbenzoic acid phenylethyl alcohol ester obtained in the above-mentioned Example 2 in a pre-dried round bottom flask, add 10 mL of anhydrous CH 2 Cl 2 , stirred, and added 1.2 equivalents of AlCl in small amounts in batches 3 , stirred at room temperature, and detected by TLC. After the reaction was complete, the reaction solution was slowly poured into 20 mL of water, extracted and separated to obtain an organic layer, and the organic layer was concentrated to dryness to obtain 2-hydroxyl-6-methoxyphenylbenzoic acid phenylethyl alcohol ester, the obtained compound Without chemical characterization, proceed directly to the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com