Human IgE (immunoglobulin E) Fab (fragment ab) against human and coding gene and application thereof
A technology of antibodies and fragments, applied in the fields of antibodies, genetic engineering, plant genetic improvement, etc., can solve problems such as adverse reactions, achieve the effects of inhibiting allergic reactions, mast cell degranulation and histamine release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Construction of Natural Human Immunoglobulin Gene Library
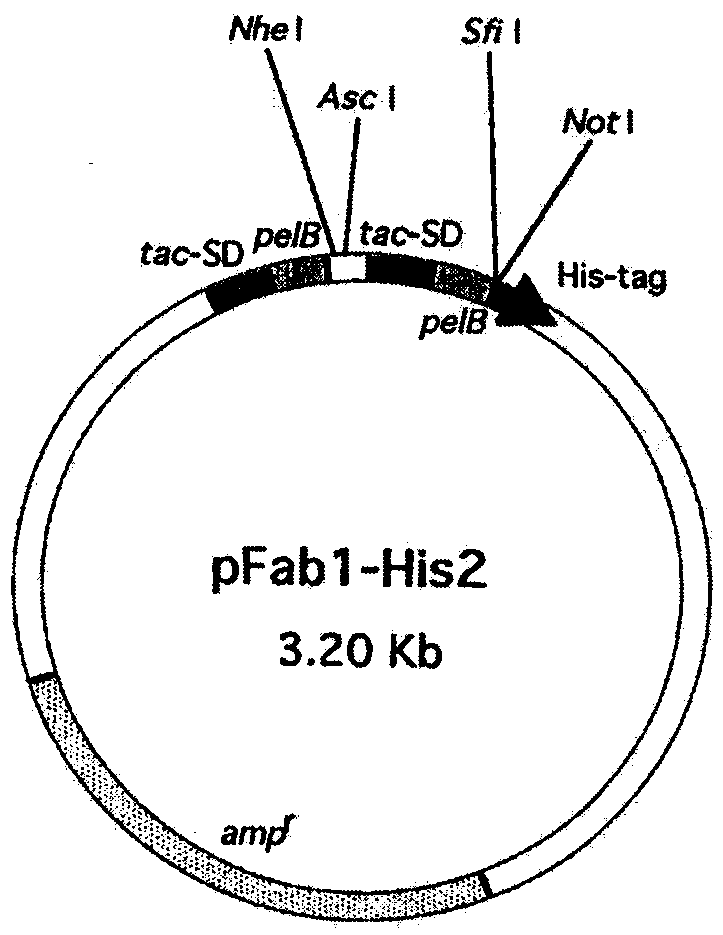
[0038] Get the peripheral anticoagulant blood of 300 healthy people (every person 5ml), separate lymphocyte with Ficoll-Paque (Pharmacia, Uppsala, Sweden), extract total RNA with kit (QIAGEN GmbH, Hilden, Germany); Gene-Amp RNA PCR Kit (Perkin-Elmer Cetus, Norwalk, Conn) was reverse-transcribed into cDNA with Oligo (dT) 16, and the upstream and downstream primers (Invitrogen) of the conserved sequence of the human IgG light and heavy chain variable region (Table 1) 1) for PCR amplification of immunoglobulin γ, κ, λ chain genes; the PCR products were purified by a kit (QIAGEN GmbH, Hilden, Germany), and then digested with AscI and NheI (NEW ENGLAND BioLabs) respectively. chain and lambda chain products. The κ chain and λ chain products after digestion were combined with the human immunoglobulin Fab expression vector pFab-His2 (such as figure 1shown), followed by electrotransformation into Escherichia...
Embodiment 3
[0047] Example 3. Screening of human immunoglobulin G against human IgE
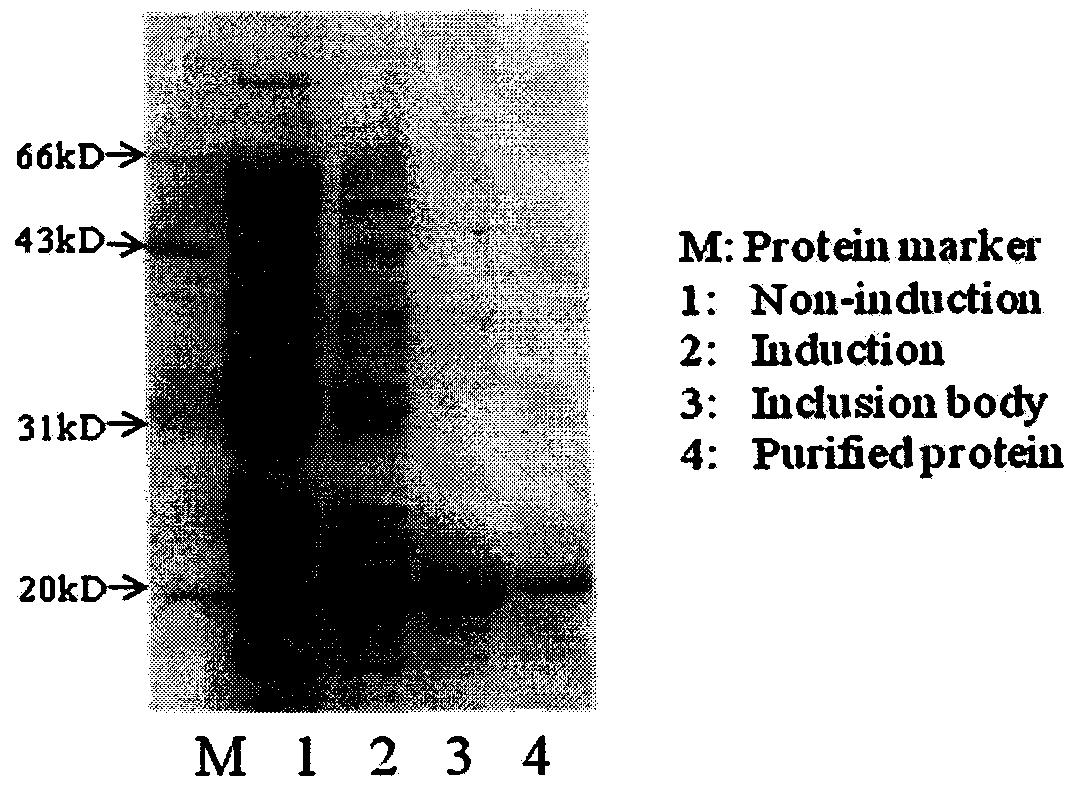
[0048] Take 9×10 7 Transform 100 μl JM109 Escherichia coli with DNA 10ng of antibody library independent of clonal titer, spread the bacterial solution on Luria broth (10g sodii chloridum, 10g tryptone, 5g yeast extract / L, PH 7) plate (containing 50 μg ampicillin / ml), cultured at 37°C for 7 hours, to be cloned (about 5×10 3 Clones / 90mm diameter plate) when the diameter is about 0.3mm, cover the plate with a nitrocellulose membrane (Armacia / Pharmacia) with a diameter of 82mm. After the clones are completely transferred to the membrane, place the membrane in LB containing 1.0mM IPTG On the plate, induce expression at 30°C for 6 hours, and then use lysozyme, DNase and bovine serum albumin (100mM Tris-HCl [pH 7], 150mM NaCl, 5mM MgCl 2 , 1.5% BSA, 1mg of DNase, 40mg lysozyme / ml) to lyse the membrane; wash to remove residual bacterial fragments etc. on the membrane, block with bovine serum albumin (BSA), a...
Embodiment 4
[0055] Example 4. Characteristic Analysis of Anti-Human IgE Antibody Fab Fragment Heavy and Light Chain Genes
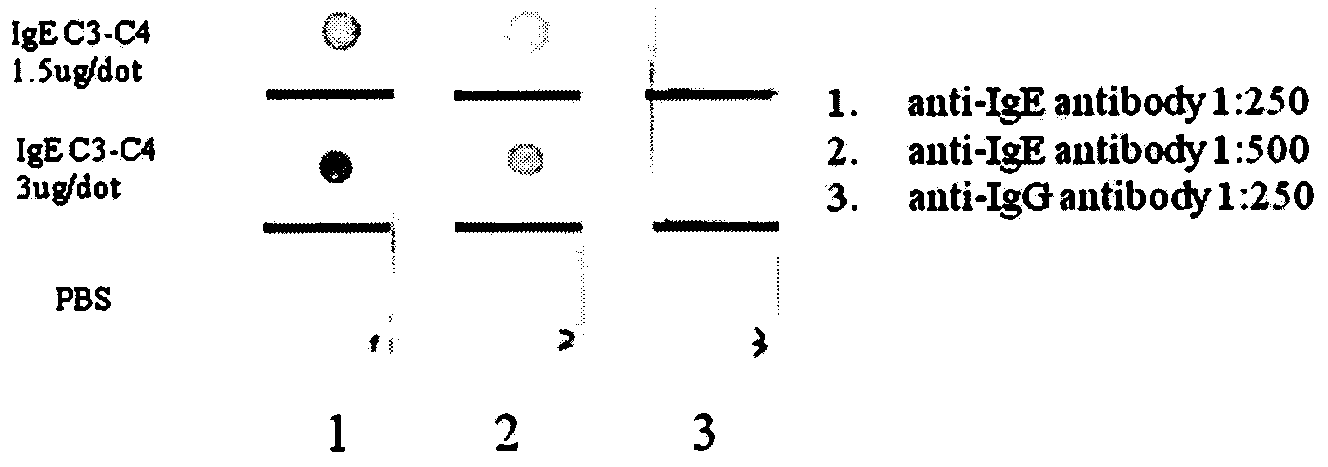
[0056] Take the plasmids of positive clones and digest them with AscI-NdeI and SfiI-NotI restriction endonucleases respectively to obtain the light chain and heavy chain genes, and then connect them with the sequencing vectors CV-2 and CV-1 modified by enzyme digestion, and transform Escherichia coli JM109, respectively extract the plasmid DNA containing the light chain or heavy chain gene, and use the M13Reverse primer (5'-GGATAACAATTTCACACAGG-3') for sequencing. The sequencing work is done by Invitrogen, and the amino acid sequence is calculated by Vector NTI 10 software , performed homology analysis with IgBlast (Table 4), and divided the CDR and FR of the light chain variable region and heavy chain variable region of positive clones according to the Kabat system (such as Figure 4 , 5 shown).
[0057] Table 4 Gene homology analysis of positive clones
[0058] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com