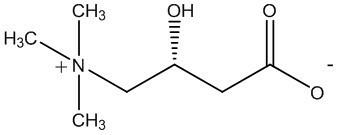
Stable levocarnitine compound
A technology of compounds and hydrates, applied in the field of stable levocarnitine compounds, which can solve problems such as affecting use and poor stability of levocarnitine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
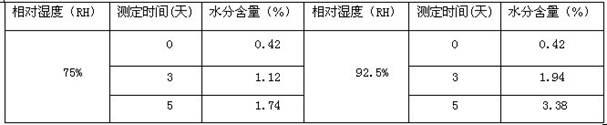
[0052]In a 2000ml reaction bottle equipped with stirring, thermometer and condenser, add 200g of levocarnitine and 1000ml of isopropanol-acetonitrile-water (5:3:2) mixture, start stirring, and heat up to 70°C -75°C, wait until it is completely dissolved, and filter while it is hot. The filtrate was naturally cooled to room temperature, and then kept warm for 8 hours to precipitate crystals, filtered, and dried indoors to obtain 181.6 grams of levocarnitine white crystals, with a melting point of 183.1°C-184.6°C, a content of 99.36%, and a specific rotation [a] 20 D is -31.1 (c=1,H 2 o). Determined by Karl Fischer method, it contains 14.30% (weight percent) of water.
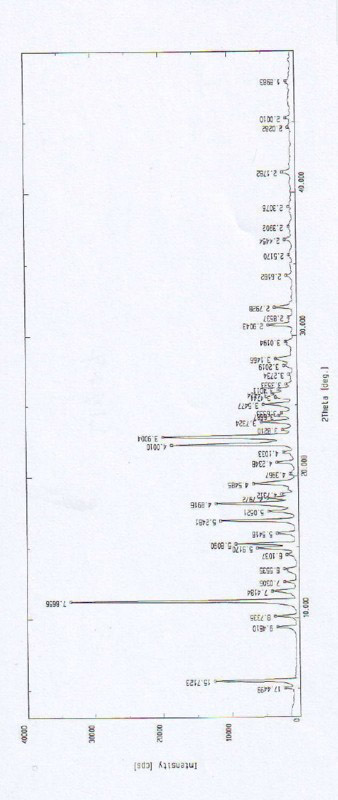
[0053] The X-ray diffraction pattern of the crystal is shown in figure 1 . Instrument model and measurement conditions: Rigaku D / max 2500 diffractometer; CuKa 40Kv 100mA; 2θ scanning range: 0-50 ° .
[0054] Microparticles or microspheres are prepared by combining the compounds of the present invention wit...
Embodiment 2
[0056] Granules containing levocarnitine sesquihydrate
[0057] Prescription: 100 grams of levocarnitine sesquihydrate, 650 grams of lactose, 80 grams of crospovidone, 50 grams of PEG-4000, 88 grams of hydroxypropyl methylcellulose, appropriate amount of distilled water, made into 1000 bags.
[0058] Process: PEG-4000 and levocarnitine sesquihydrate are crushed together, passed through an 80-mesh sieve, mixed with other materials, made into soft materials with distilled water, granulated, dried at low temperature, and then packed into granules.
Embodiment 3
[0060] Capsules containing L-carnitine sesquihydrate
[0061] Prescription: 50 grams of levocarnitine sesquihydrate, 5 ml of propylene glycol, 200 grams of starch, made into 1000 capsules.
[0062] Process: Moisten levocarnitine sesquihydrate and starch with 15% propylene glycol aqueous solution, mix well, sieve and granulate, dry at 60°C, granulate, and fill capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com