Method for synthesizing monopegylation fixed point modification thymopoietin
A technology of PEGylation and fixed-point modification, which is applied in the field of biomedicine, can solve the problems that the yield of PEGylated thymopentin is only 2%, it is difficult to remove FMOC units, and the condensation reaction of amino acids is difficult, so as to prolong the circulation in the body Time, increase bioavailability, effect of synthetic route maturity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Solid-phase synthesis of thymopentin with protective groups
[0032] A: Soak 1.5g of Fmoc-Tyr(tBu)-Wang resin (1%DVB, 100-200 mesh, S=0.3mmol / g) in dichloromethane (DCM) for 30 minutes, drain, and use dimethylformamide (DMF) after washing (three washes, three minutes each); add a mixed solution of piperidine and dimethylformamide (DMF) (piperidine / dimethylformamide = 1 / 4 (v / v)) Add to the washed resin, stir mechanically for 30 minutes, remove the Fmoc protecting group, wash with DMF (wash three times, each time for three minutes), and obtain the deprotected resin Tyr(tBu)-Wang. Add indene detection reagent (5% ninhydrin alcohol solution), heat in boiling water for three minutes, blue color appears, and the Fmoc group is completely removed.
[0033]B: amino acid FMOC-Val-OH 458mg (1.35mmol, 3eq)), benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU) 512.06mg (1.35mmol ,3eq), 1-hydroxybenzotriazole (HoBt) 182.66mg (1.35mmol, 3eq), dissolved in DMF i...
Embodiment 2
[0054] (1) Solid-phase synthesis of thymopentin with protective groups
[0055] A: Same as Example 1.
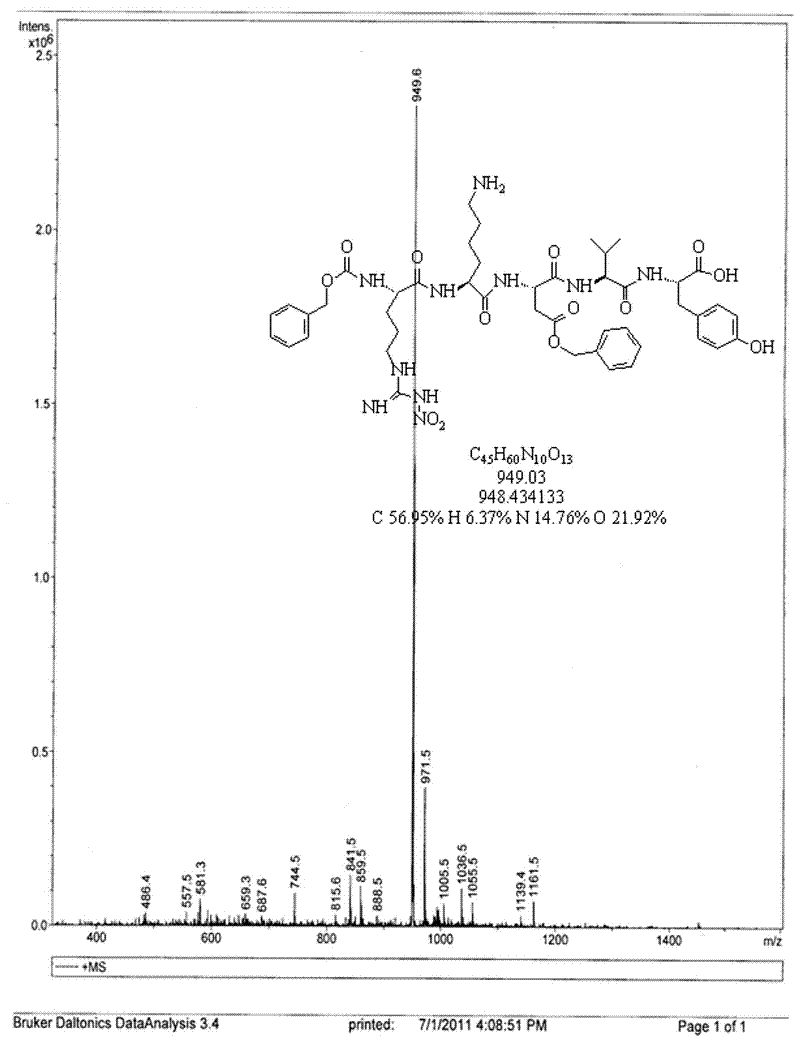
[0056] B: Change the amount of amino acid to FMOC-Val-OH: 305mg (0.9mmol, 2eq), FMOC-Asp(oBzl)-OH: 401mg (0.9mmol, 2eq), FMOC-Lys(BOC)-OH: 422mg (0.9 mmol, 2eq), Z-Arg (NO 2 )-OH: 318mg (0.9mmol, 2eq), benzotriazole-N, N, N', N'-tetramethyluronium hexafluorophosphate (HBTU): 170mg (0.45mmol, 1eq), 1- Hydroxybenzotriazole (HoBt): 61mg (0.45mmol, 1eq), the others are the same as in Example 1, and the thymopentin Z-Arg (NO 2 )-lys-Asp(oBzl)-Val-Tyr pure product 245.8mg, yield 53.83%.
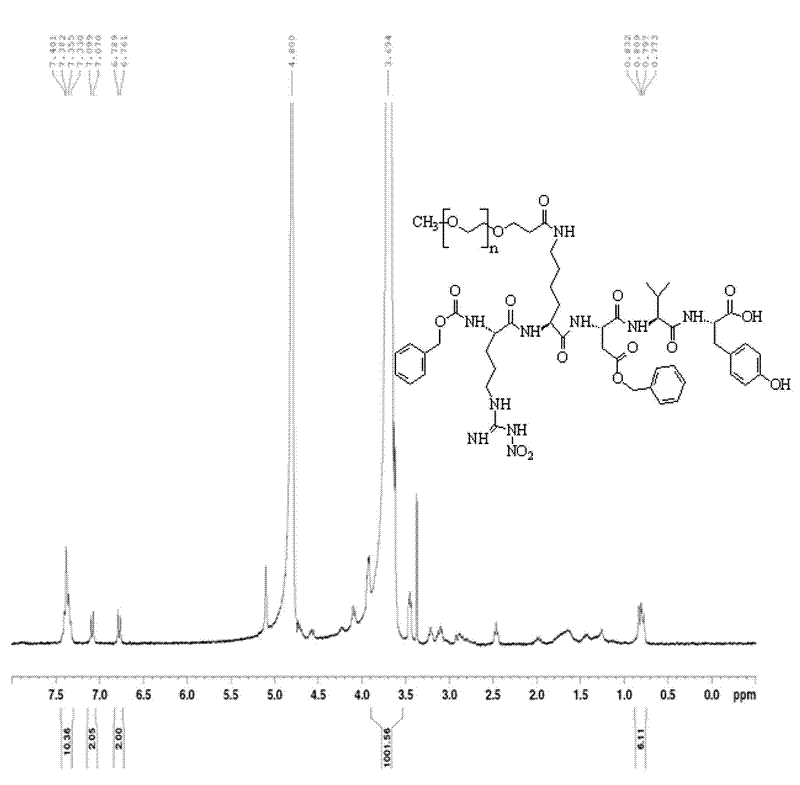
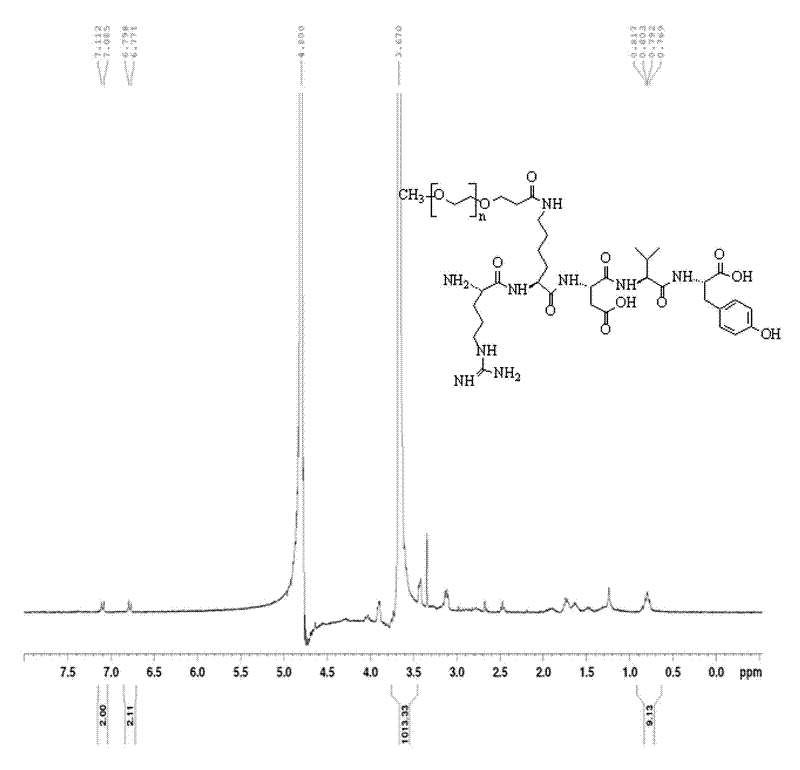
[0057] (2) Liquid phase synthesis of PEGylated Thymopentin
[0058] Thymopentin Z-Arg (NO 2The amount of )-lys-Asp(oBzl)-Val-Tyr pure product is 42.7mg (3eq, 0.045mmol), the monomethoxypolyethylene glycol modification (mPEG-SPA) is 150mg (0.015mmol, 1eq), Others are identical with embodiment 1, get Z-Arg (NO 2 )-lys(mPEG)-Asp(oBzl)-Val-Tyr pure product 91.48mg, yield rate 55.7%.
[0059] ...
Embodiment 3
[0062] (1) Solid-phase synthesis of thymopentin with protective groups
[0063] A: Same as Example 1.
[0064] B: Change the amount of amino acid to FMOC-Val-OH: 611mg (1.8mmol, 4eq), FMOC-Asp(oBzl)-OH: 801mg (1.8mmol, 4eq), FMOC-Lys(BOC)-OH: 843mg (1.8 mmol, 4eq), Z-Arg (NO 2 )-OH: 635mg (1.8mmol, 4eq), benzotriazole-N, N, N', N'-tetramethyluronium hexafluorophosphate (HBTU): 341mg (0.9mmol, 2eq), 1- Hydroxybenzotriazole (HoBt): 122mg (0.9mmol, 2eq), the others are the same as in Example 1, and the thymopentin Z-Arg with a protective group is finally obtained (NO 2 )-lys-Asp(oBzl)-Val-Tyr pure product 265mg, yield 62.12%.
[0065] (2) Liquid phase synthesis of PEGylated Thymopentin
[0066] Thymopentin Z-Arg (NO 2 The amount of )-lys-Asp(oBzl)-Val-Tyr pure product is 189.6mg (0.2mmol, 20eq), the monomethoxypolyethylene glycol modification (mPEG-SPA) is 100mg (0.01mmol, 1eq), Others are identical with embodiment 1, get Z-Arg (NO 2 )-lys(mPEG)-Asp(oBzl)-Val-Tyr pure prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com