Preparation method of vitamin K2 compounds
A compound and metal compound technology, applied in the field of organic drug synthesis, can solve problems such as unsuitable for industrial production, harsh conditions, and cumbersome operations, and achieve the effects of easy separation and purification, mild reaction conditions, and reduced pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
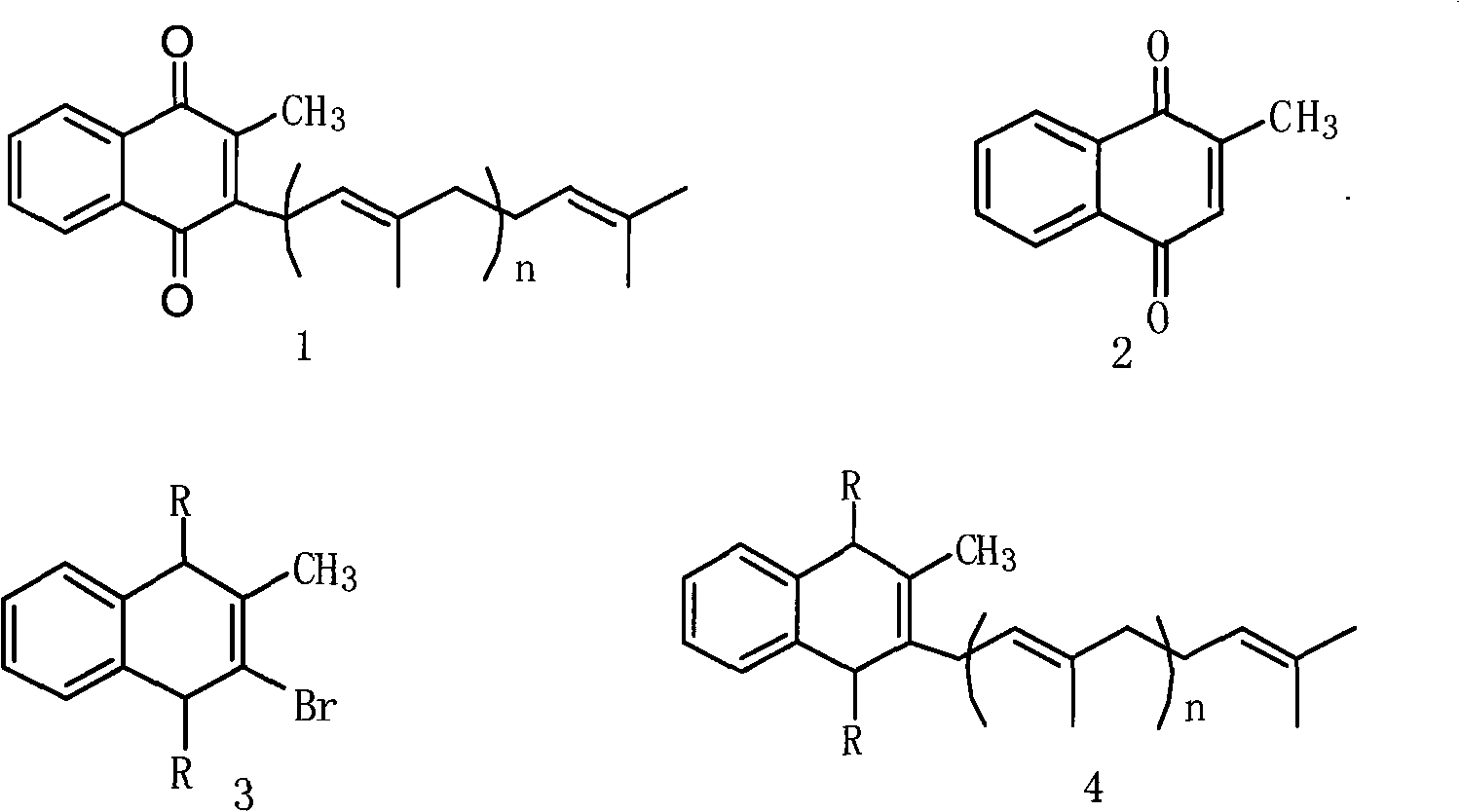
[0050] (1) Synthesis of compound shown in formula 3
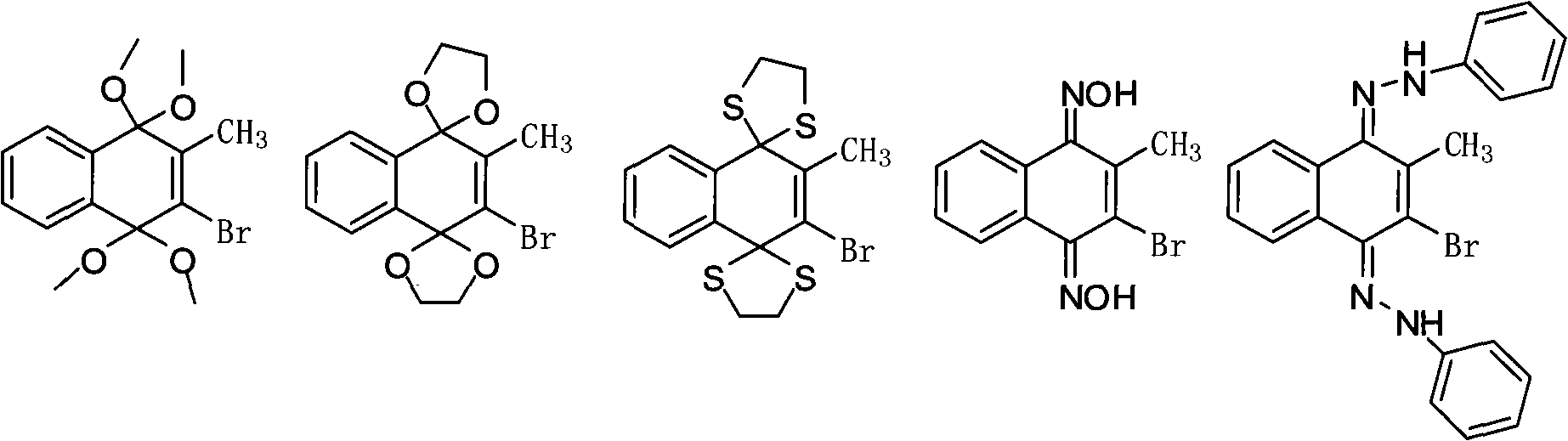
[0051] Add 500ml of methanol and 100g of 2-methyl-1,4-naphthoquinone into a 1L four-neck flask, cool down to 0°C, add 111.7g of bromine dropwise at a temperature of 0-10°C, and pay attention to connecting the alkali absorption device. After dripping, keep warm at 10-30°C for 3-5 hours, slowly heat up to reflux and react for 3 hours, cool down to room temperature naturally, then cool to 0-5°C for natural crystallization for 10 hours, suction filter, reduce at 25-30°C Drying under pressure gave 124.4 g of white solid with a yield of 91%.
[0052] (2) Synthesis of compound shown in formula 4
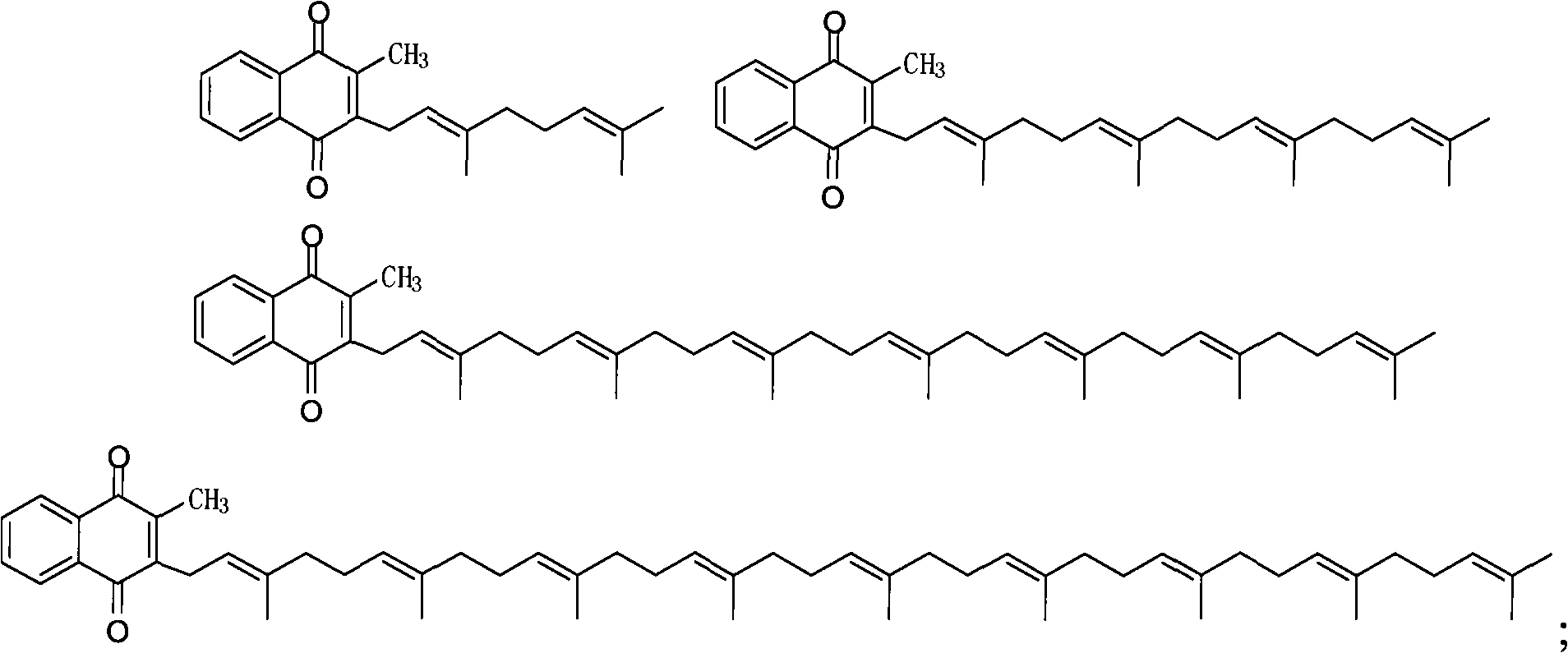
[0053] A. Add 87g of tri-n-butyltin hydrogen and 200ml of tetrahydrofuran into a 1L dry four-necked bottle, cool down to -10°C, and add 100g of geranylgeranyl bromide in 300ml of tetrahydrofuran solution dropwise at -10°C to 10°C. Warm to 20-25°C until the reaction is complete, and filter until dry to obtain 163.2 g of a yellow solid, ...
Embodiment 2
[0058] (1) Synthesis of compound shown in formula 3
[0059] Add 500ml of toluene, 100g of 2-methyl-1,4-naphthoquinone, 49.5g of ethylene glycol into a 1L four-neck flask, and after refluxing for 3 hours, cool down to 0°C, and dropwise add the Phosphorus oxybromide 200g tetrahydrofuran solution 100ml, pay attention to connect the alkali absorption device. After dripping, keep warm at 10-30°C for 3-5 hours, slowly heat up to reflux and react for 3 hours, cool down to room temperature naturally, then cool to 0-5°C for natural crystallization for 10 hours, suction filter, reduce at 25-30°C Drying under pressure gave 120.3 g of white solid with a yield of 89%.
[0060] (2) Synthesis of compound shown in formula 4
[0061] A. Add 50g of elemental magnesium, 1g of elemental iodine, 600ml of tetrahydrofuran and 100g of geranylgeranyl bromide into a 1L dry four-necked bottle, slowly heat to reflux for 10h, and naturally cool down to room temperature, that is, the geranylgeranyl magn...
Embodiment 3
[0066] (1) Synthesis of compound shown in formula 3
[0067] Add 500ml of toluene, 100g of 2-methyl-1,4-naphthoquinone, and 75.5g of ethanedithiol into a 1L four-neck flask. After refluxing for 3 hours, cool down to 0°C and add dropwise at 0-10°C. 100ml of tetrahydrofuran solution containing 200g of phosphorus oxybromide, pay attention to connect the alkali absorption device. After dripping, keep warm at 10-30°C for 3-5 hours, slowly heat up to reflux and react for 3 hours, cool down to room temperature naturally, then cool to 0-5°C for natural crystallization for 10 hours, suction filter, reduce at 25-30°C Drying under pressure gave 143 g of white solid with a yield of 89%.
[0068] (2) Synthesis of compound shown in formula 4
[0069] A. Add 50g of elemental zinc, 1g of elemental iodine, 600ml of toluene and 100g of geranylgeranyl bromide into a 1L dry four-necked bottle, slowly heat to reflux for 10 hours, and naturally cool down to room temperature, that is, the geranylg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com