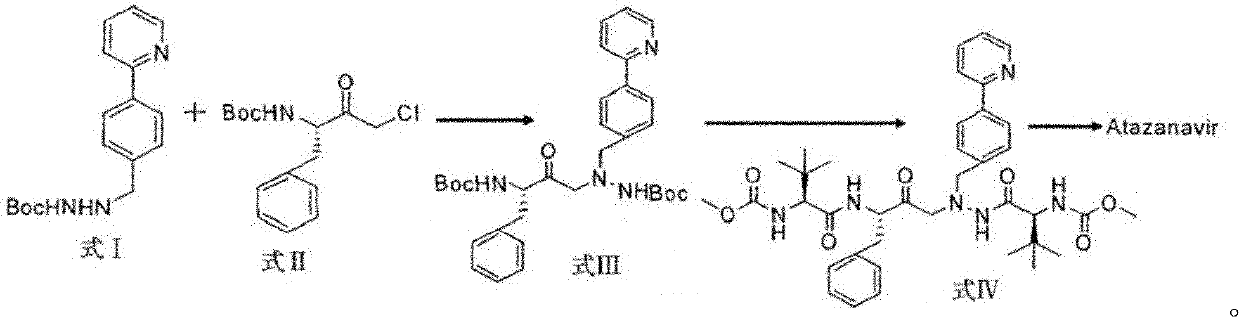
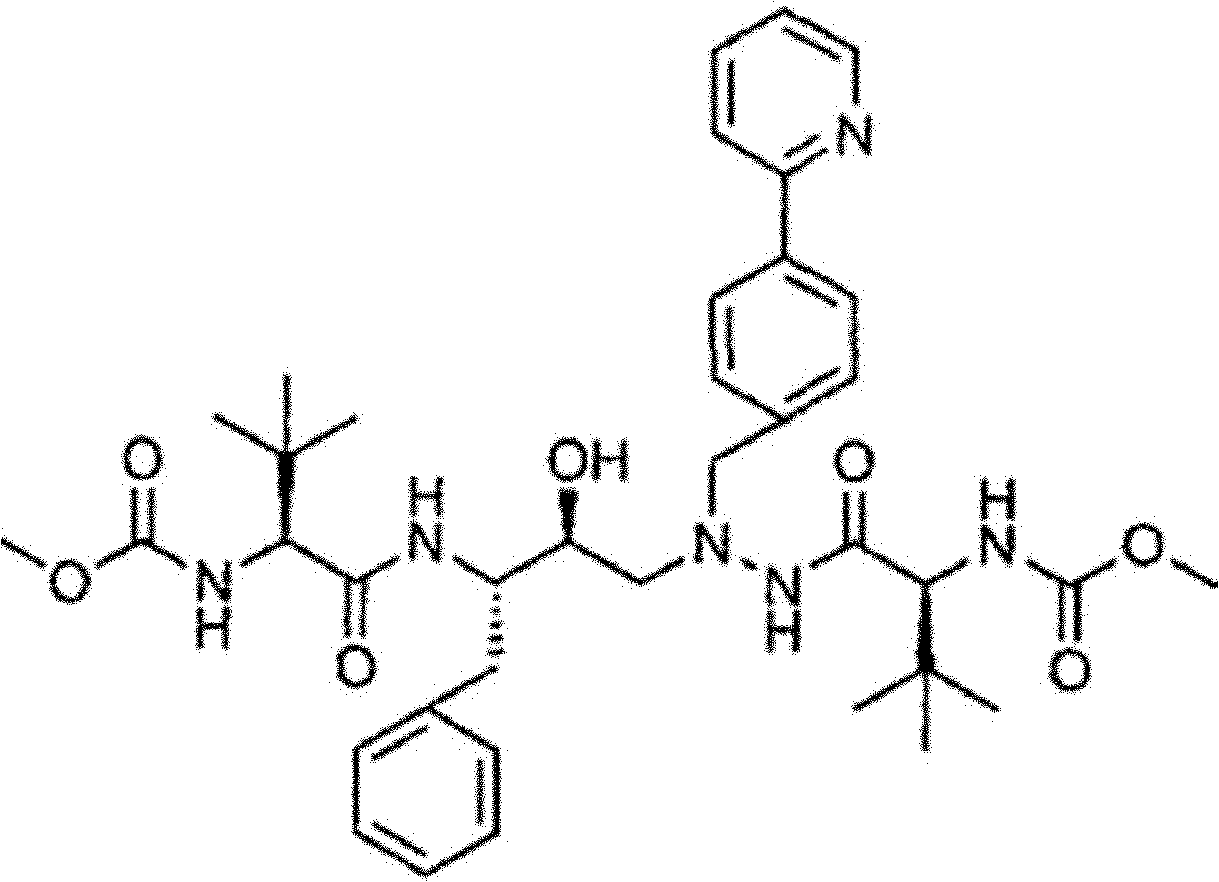
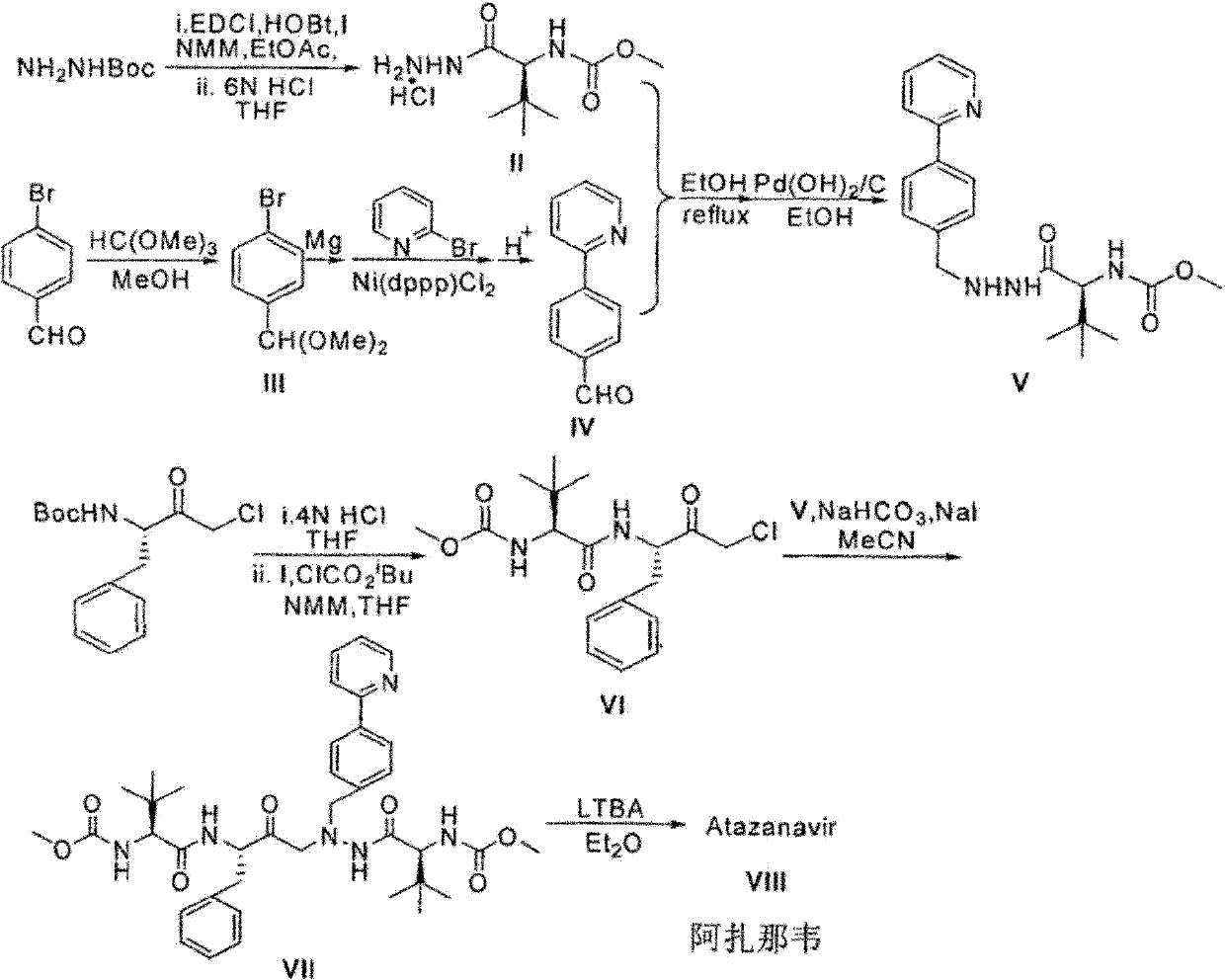
Novel method for synthesizing Atazanavir
A new method and compound technology, which is applied in the field of chemical drug synthesis, can solve the problems of unsuitable industrial production requirements, many synthesis steps, and high total cost, and achieve the effects of low price, simple purification treatment, and reduced total cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: synthetic formula I compound
[0034]
[0035] a) Synthesis of compound 1:
[0036] Add p-bromobenzaldehyde (37g, 0.2mol) into methanol (500ml) dissolved with trimethyl orthoformate (31.8g, 0.3mol) and p-toluenesulfonic acid (0.38g, 2mmol), and stir at room temperature for 3 hours Sodium methoxide (0.43 g, 8 mmol) was added, the solution was concentrated after stirring for 1 hour, and the colorless oily liquid 1 was obtained by filtration, 44.96 g, yield 98%. 1 HNMR (CDCl 3 )δ: 7.49 (d, 2H, J = 8.7Hz), 7.33 (d, 2H, J = 8.7Hz), 5.36 (s, 1H), 3.30 (s, 6H).
[0037] b) Synthesis of compound 2:
[0038] Add compound 1 (44.89g, 195mmol) into tetrahydrofuran (40ml) dissolved with magnesium chips (5.11g, 212mmol), add a small amount of iodine, initiate under heating, and the solution turns brown; stir for 1 hour after initiation; Add the Grignard reagent dissolved in dibromopyridine (17.14ml, 177mmol) and Ni[dppp]Cl under ice bath 2 (1 g) in tetrahydrofur...
Embodiment 2
[0045] Embodiment 2: synthetic formula III compound
[0046]
[0047] At room temperature, sodium iodide (12.4g, 83mmol) was added into acetonitrile (250ml) dissolving 76mmol of compound of formula II (250ml), stirred for 30 minutes and then added MeCN solution (350ml) of 83mmol of compound of formula I dissolved, and continued to stir for 30 minutes , add sodium bicarbonate (12.7g, 152mmol), stir at room temperature for 12 hours and remove acetonitrile under reduced pressure, the system is diluted with ethyl acetate (800ml), washed twice with saturated potassium hydrogensulfate (300ml), washed with saturated brine (600ml) Wash once, dry, and concentrate to obtain the crude product, reflux with ethanol / water=1:2, the system gradually becomes transparent, cool down to room temperature, spin dry the ethanol under reduced pressure, extract the aqueous phase with ethyl acetate (200ml) three times, The organic phases were combined, dried and concentrated to obtain the compound o...
Embodiment 3
[0048] Embodiment 3: Synthesis of N-methoxycarbonyl-L-tert-leucine
[0049]
[0050] a) Synthesis of Compound 5:
[0051] Under ice bath, 3,3-dimethyl-butan-2-one (62.5ml, 0.5mol) was slowly added dropwise into a solution of potassium permanganate (158g, 1mol), sodium hydroxide (50g, 1.25mol) Aqueous solution (1L), stirred at 0°C for 1 hour, heated to 25°C and stirred for 2 hours; filtered the mixture with suction, washed the manganese dioxide residue with a small amount of hot water for 3 times, combined the filtrate and washing liquid, concentrated to 200ml, adjusted with 6N hydrochloric acid The concentrated solution was adjusted to a pH value of 3, extracted three times with ethyl acetate (100ml), combined the extracts, and dried with anhydrous sodium sulfate; the diethyl ether was drained, and the resulting oily product was distilled under reduced pressure, and fractions at 100°C / 30225kPa were collected to obtain Color oily liquid 5, 45.45g, yield 70%. 1 HNMR (CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com