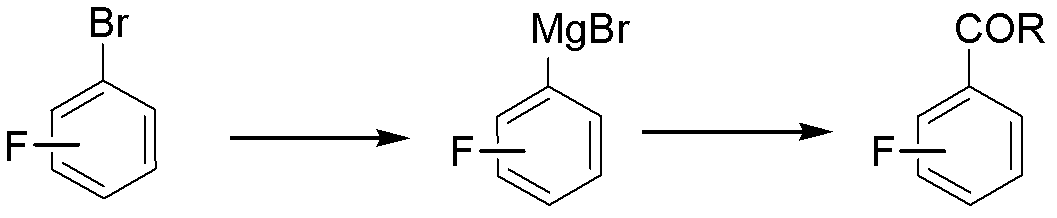
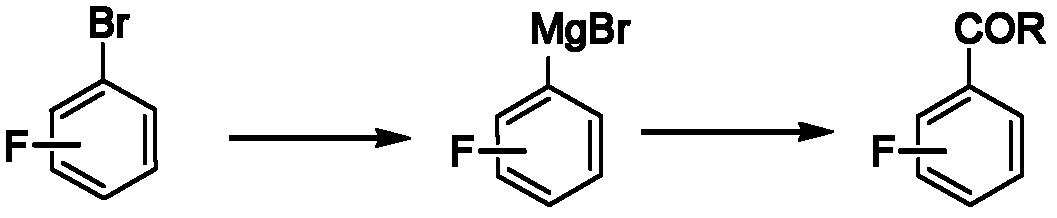
Method for preparing fluorine-containing substituted phenyl ketone
A technology of phenyl ketone and fluorobromobenzene, which is applied in the field of preparing halogenated phenyl ketones, can solve the problems of long and complicated operation, instability, harsh reaction temperature control, etc., and achieves a simple process, high conversion rate and mild conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A kind of preparation 3,5-difluoroacetophenone The method is characterized in that the specific preparation steps are as follows:
[0026] (1) Add 712kg (5vol) of tetrahydrofuran (THF) and 28kg (1.4eq) of magnesium powder to a 2000L reactor, and dropwise add 445kg of tetrahydrofuran (2vol) solution containing 160kg of main raw material 3,5-difluorobromobenzene to prepare the Grignard reagent 3,5-Difluorophenylmagnesium bromide;
[0027] (2) Add 427kg (3vol) of THF, 5.7kg (0.07eq) of cuprous chloride, 7.7kg (0.07eq) of aluminum trichloride, and 169.3kg (2.0eq) of acetic anhydride to another 3000L reactor. Add the above-mentioned Grignard reagent 3,5-difluorophenylmagnesium bromide dropwise at 10±2°C, and react at this temperature for 4 hours after dropping;
[0028] (3) After the reaction is completed, extract, wash the organic phase with brine, and concentrate to obtain the product 3,5-difluoroacetophenone 106kg, yield 82.0%, gas chromatography purity (GC) 99.8%. ...
Embodiment 2
[0031] A kind of preparation o-fluoropropiophenone The method is characterized in that the specific preparation steps are as follows:
[0032] (1) Add 51.6kg (3vol) of 2-methyltetrahydrofuran (2-methyltetrahydrofuran) and 3.3kg (1.2eq) of magnesium powder to a 200L reactor, and dropwise add 17.2kg (1vol) of 2-methyltetrahydrofuran (2-methyltetrahydrofuran) containing 20kg of o-fluorobromobenzene as the main raw material Solution 37.2kg, prepared Grignard reagent o-fluorophenylmagnesium bromide;
[0033] (2) Add 2-methyltetrahydrofuran 34.4kg (2vol) successively in another 300L reactor, iron trichloride 0.9kg (0.05eq), aluminum trichloride 0.8kg (0.05eq), propionic anhydride 22.3kg ( 1.5eq), add the above-mentioned Grignard reagent o-fluorophenylmagnesium bromide dropwise under temperature control at 5±2°C, and react at this temperature for 3 hours after dropping;
[0034] (3) After the reaction is completed, extract, wash the organic phase with brine, and concentrate to obt...
Embodiment 3
[0037] A kind of preparation 2,3-difluorobutyrophenone The method is characterized in that the specific preparation steps are as follows:
[0038](1) add methyl tert-butyl ether 237kg (8vol) in 1000L reactor, magnesium powder 9kg (1.8eq), add dropwise the methyl tert-butyl ether containing main raw material 2,3-difluorobromobenzene 40kg ( 4vol) solution 159kg, prepared Grignard reagent 2,3-difluorophenylmagnesium bromide;
[0039] (2) Add 207kg (7vol) of methyl tert-butyl ether, 1.8kg (0.1eq) of manganese dioxide, 2.7kg (0.1eq) of aluminum trichloride, and 82kg (2.5eq) of butyric anhydride to another 1000L reactor. ), add the above-mentioned Grignard reagent 2,3-difluorophenylmagnesium bromide dropwise under temperature control at 15±2°C, and react at this temperature for 6 hours after dropping;
[0040] (3) After the reaction is completed, extract, wash the organic phase with brine, and concentrate to obtain the product 2,3-difluorobutyrophenone 30.4kg, yield 79.9%, gas ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com