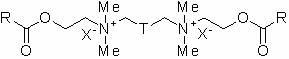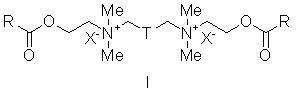Atom transfer free radical polymerization initiator with gemini surface activity and synthetic method thereof
A technology of gemini surface activity and polymerization initiator, applied in the field of chemistry, can solve the problems of poor water resistance of polymers, inconvenient operation, affecting the optical, electrical and surface properties of products, etc. body wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 N 1 ,N 6 -Bis(2-(2-bromoisobutyryloxy)ethyl)- N 1 ,N 1 ,N 6 ,N 6 -Tetramethylhexane-1,6-diammonium bromide (compound I-a )Synthesis
[0025] Add 20 mL (0.3 mmol) dichloromethane (DCM), 4.45 g (50 mmol) N,N - Dimethylethanolamine, 9 mL (63 mmol) triethylamine (TEA). Magnetic stirring, fully stirring to dissolve and mix well. At room temperature, 7.5 mL (60 mmol) of 2-bromoisobutyryl bromide was slowly added dropwise to the reaction solution. After dripping, react for 2 h. Filter to remove solids. After the filtrate was concentrated, ethyl acetate was added to dissolve it, washed with water, and saturated with Na 2 CO 3 Wash, anhydrous Na 2 SO 4 Dry and distill under reduced pressure to obtain the product II-a (9.05 g, 86.1%). 1 H NMR (400 MHz, CDCl 3 ): δ 4.29 (2H, d, J= 4 Hz), 2.64 (2H, d, J = 4 Hz), 2.32 (6H, s), 1.94 (6H, s); 13 C NMR (100 MHz, CDCl 3 ): δ 171.5, 64.1, 57.2, 55.7, 45.7, 30.7;
[0026] Under nitrogen protection,...
Embodiment 2
[0031] Example 2 N 1 ,N 4 - Bis[(4-chloromethylbenzoyloxy)ethyl]- N 1 ,N 1 ,N 4 ,N 4 -Tetramethylhexane-1,4-diammonium bromide (compound I-b ) Synthesis
[0032] 4-Chloromethylbenzoic acid (10.53 g, 60 mmol) was dissolved in thionyl chloride (40 mL), and a catalytic amount of N,N -Dimethylformamide (DMF), refluxed for 5 h, concentrated. The residue was dissolved in anhydrous tetrahydrofuran (20 mL), and added dropwise to N,N -Dimethylethanolamine (4.45 g, 50 mmol) and pyridine (4.98 g, 63 mmol) in THF. After dropping, react at room temperature for 3 h. Then proceed in a similar manner to Example 1 to obtain an intermediate II-b (9.21 g, 76.2%). 1 H NMR (400 MHz, CDCl 3 ): δ 8.04 (d, 2H), 7.46 (d, 2H), 4.62 (s, 2H), 4.28 (d, 2H), 2.64 (d, 2H), 2.32 (s, 6H); 13 C NMR (100 MHz, CDCl 3 ): δ 166.1, 142.3, 130.3, 130.0, 128.4, 64.2, 57.2, 55.7, 46.3, 45.7;
[0033] Under nitrogen protection, the intermediate II-b (3.38 g, 14 mmol), 10 mL of acetone...
Embodiment 3
[0038] Example 3 N- {4-[((2-bromoisobutyryloxy)ethyl)dimethylaminomethyl]benzoylbenzyl}-2-(2-bromoisobutyryloxy)- N,N -Dimethylethaneammonium bromide (compound I-c ) Synthesis
[0039] First prepare the intermediate according to the same method as in Example 1 II-a ,
[0040] 4,4'-Bis(bromomethyl)benzophenone was then prepared. Dissolve 4,4'-dimethylbenzophenone (1.5 g, 7.1 mmol) and carbamide peroxide (1.47 g, 15.7 mmol) in dichloromethane (20 mL), reflux, and illuminate with a 100 W incandescent lamp , then hydrobromic acid (2.9 mL, 14.3 mmol) was added dropwise, and the reaction was continued for 30 min after the drop was completed. Cool, add deionized water to the reaction solution, separate the liquids, dry the organic phase with anhydrous sodium sulfate, filter, and spin dry the solvent to obtain a white solid, then recrystallize with absolute ethanol to obtain the product 4,4'-bis(bromo Methyl)benzophenone (1.20 g, 46%). M.p. 120-122 o C.IR: ν (cm -1 ) 305...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com