Synthesis method of in-situ-enhanced thermosensitive polymer and degradable in-situ-enhanced injectable thermosensitive hydrogel
A temperature-sensitive hydrogel and synthesis method technology, applied in the field of in-situ enhanced synthesis of injectable temperature-sensitive hydrogel, can solve the problems of cell damage, tissue necrosis, cell death, etc., and achieve the effect of enhanced strength and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
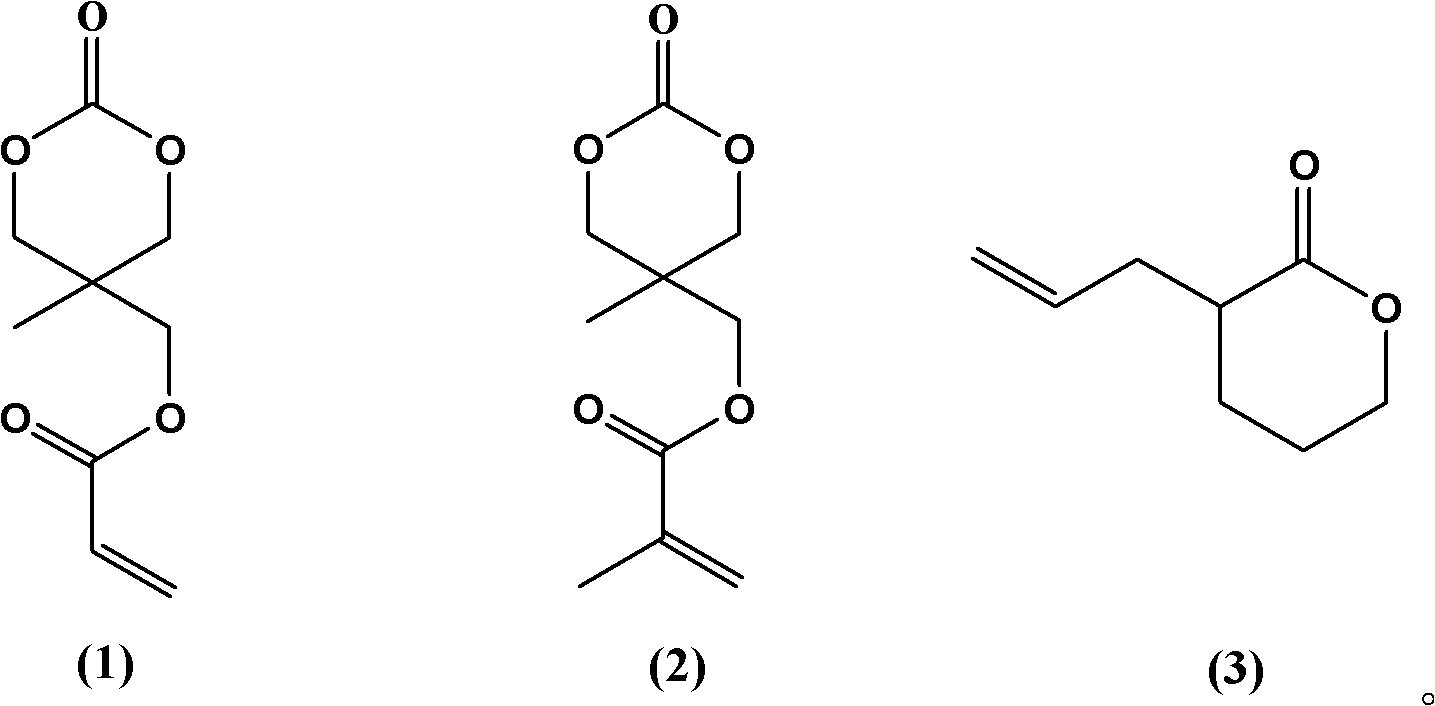
[0026] A method for synthesizing a temperature-sensitive polymer capable of in situ enhancement, comprising the following steps: in the presence of a hydroxyl group-containing initiator and a catalyst, homopolymerize a cyclic lactone functional monomer containing a double bond, or obtain it from a double-bond-containing functional monomer The cyclic lactone functional monomer is obtained by copolymerization with one of carbonate, lactone and lactide, wherein the double bond functional monomer is a compound shown in structural formula (1)-(3):
[0027]
[0028] The hydroxyl group-containing initiator is preferably Pluronic F127, PLGA-PEG-PLGA, unless otherwise specified, the F127 mentioned below is Pluronic F127.
[0029] Described catalyst is a kind of in metal or metalloid catalyst; Metal catalyst comprises stannous octoate, tin trifluoromethanesulfonate; Nonmetal catalyst comprises aliphatic tertiary amine (comprising triethylamine (TEA), N, N, N',N'-tetramethylethylenedi...
Embodiment 1
[0039] Weigh 1.89g (1.5×10 -4 mol) F127 was dehydrated in a reaction polymerization tube at 120° C. for 4 h under vacuum, protected by Ar and cooled to room temperature. in a vacuum glove box (O 2 Content2 O content -4 mol) TMEDA (N, N, N', N'-tetramethylethylenediamine), 0.364 (3.57×10 -3 mol) TMC (trimethylene carbonate), 0.126g (6.3×10 -4 mol) AC (2-methyl 2-methyl acrylate-1,3 dimethylene carbonate). The polymerization tube was sealed, and after reacting at 60° C. for 45 h, the reaction polymerization tube was placed in an ice bath to terminate the polymerization reaction. The crude product was precipitated using ice anhydrous diethyl ether 8-10 times the volume of THF at room temperature. The anhydrous ether precipitate was then dissolved in THF, dried under reduced pressure at 40°C to constant weight, and stored in a desiccator.
Embodiment 2
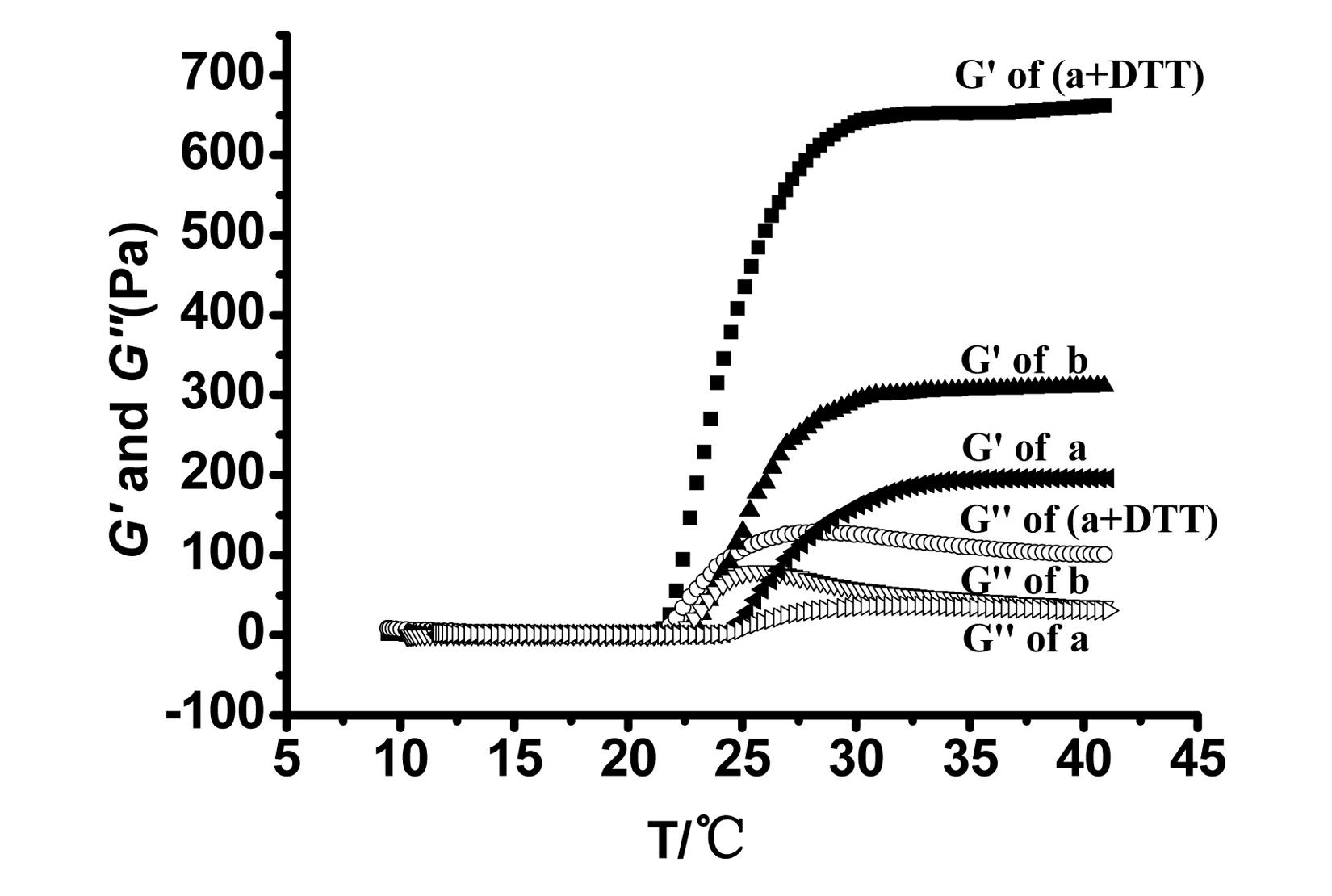
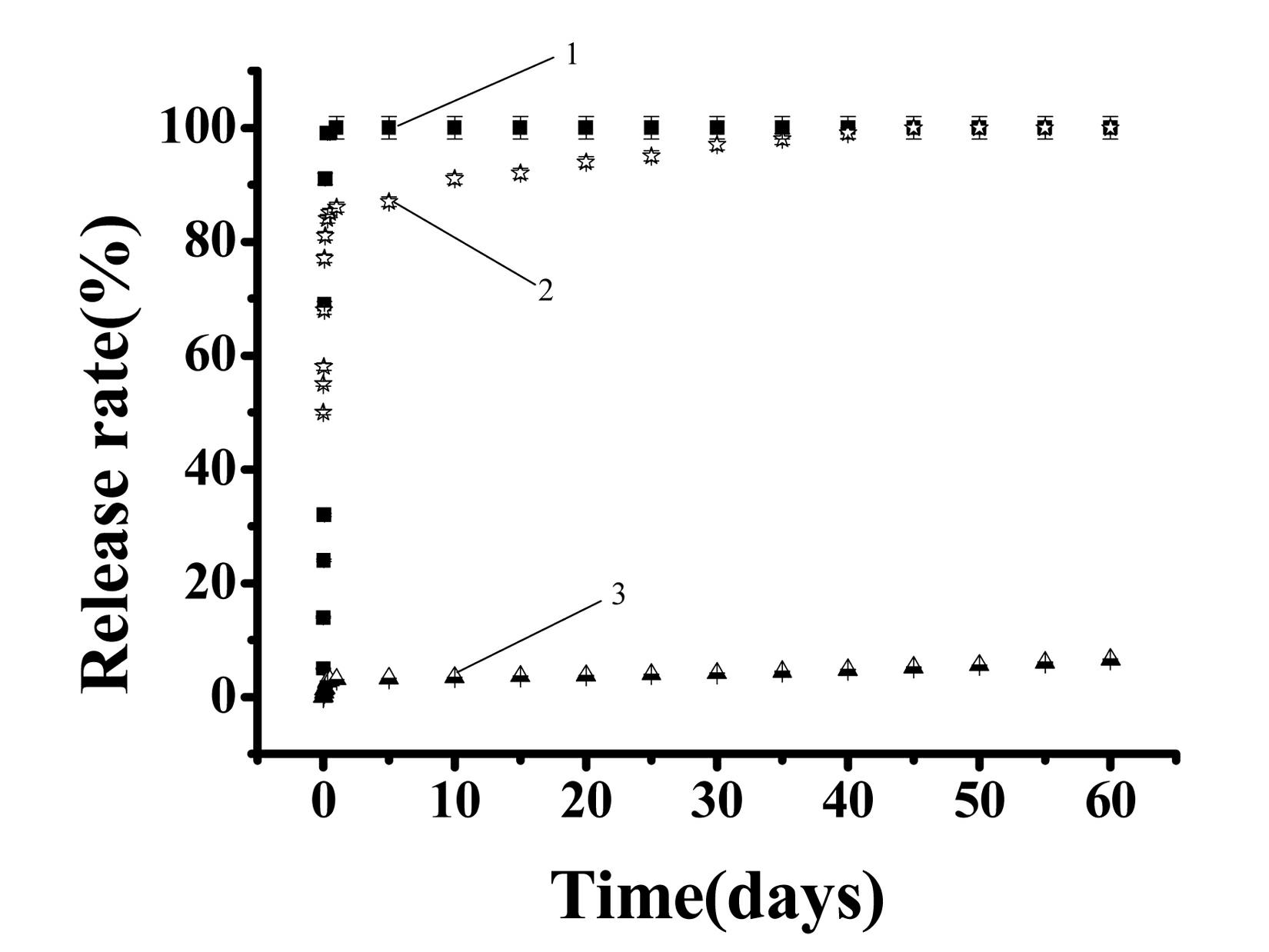
[0041] Dissolve 0.06 g of the copolymer obtained in Example 1 in 1 ml of PBS (pH=7.4), and place it in a refrigerator at 4°C for overnight storage, so that the aqueous solution of the hydrogel material reaches an equilibrium state. Add 1mgDTT (dithiothreitol) to the aqueous solution; and react at 40°C for 10min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com