Titanium barium phosphate salt, and preparation method and application thereof
A technology of barium titanium phosphate and barium titanium phosphate, which is applied in the field of inorganic scintillating luminescent materials, can solve problems such as unreported applications, and achieve the effects of simple preparation process, simple equipment, and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of Ba 2 TiP 2 o 9 , the steps include: weighing barium carbonate BaCO 3 : 7.89 g, Titanium Dioxide TiO 2 : 1.60 g, ammonium dihydrogen phosphate NH 4 h 2 (PO 4 ): 3.961 g, after grinding and mixing evenly, select the air atmosphere for the first calcination, the temperature is 300 ° C, the calcination time is 8 hours, then cool to room temperature, and take out the sample. Fully mix the mixture and grind it evenly, then calcine it for the second time in the air atmosphere, the temperature is 800°C, and the calcination time is 1.5 hours, then fully mix the mixture and grind it evenly, and then sinter it for the third time in the air atmosphere at 1550°C, the sintering time is 2 hours, and then cooled down to 900°C at a rate of 10°C per hour, and then automatically cooled down to room temperature to obtain bulk titanium barium phosphate scintillation materials.
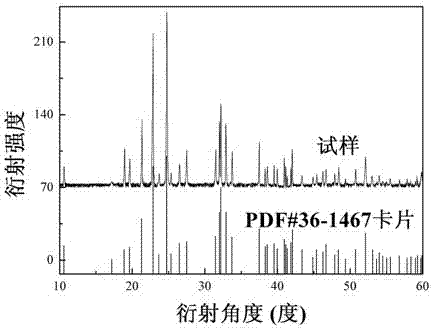
[0034] See attached figure 1 , which is a comparison between the X-ray powder diffraction ...
Embodiment 2
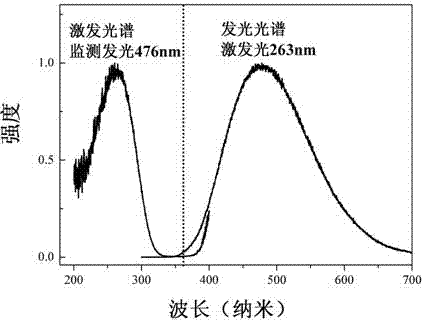
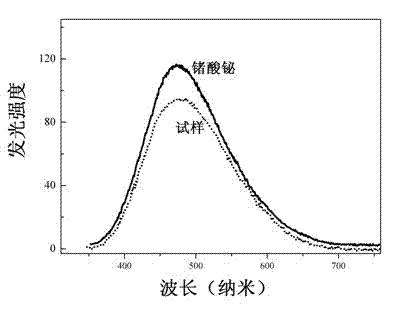
[0040] Preparation of BaSrTiP 2 o 9 , the steps include: weighing barium carbonate BaCO 3 : 3.945 g, strontium carbonate SrCO 3 : 2.953 g, Titanium Oxide TiO 2 : 1.60 g, ammonium dihydrogen phosphate NH 4 h 2 (PO 4 ): 3.961 g, after grinding and mixing evenly, select the first calcination in the air atmosphere, the temperature is 350 ° C, the pre-sintering time is 6 hours, and cool to room temperature. Take out the sample, grind it thoroughly again and mix it evenly, calcinate it for the second time, and sinter it at 1500°C for 1.5 hours in an air atmosphere, then cool it down to 850°C at a rate of 15°C per hour, and then cool it down to room temperature automatically to get Bulk barium titanium phosphate scintillation material. The main structural properties, X-ray excitation spectrum, excitation spectrum and luminescence spectrum are similar to those of Example 1.
Embodiment 3
[0042] Preparation of Ba 1.9 Ca 0.1 TiP 2 o 9 , the steps include: weighing barium carbonate BaCO 3 : 3.551 g, calcium carbonate CaCO 3 : 0.2 g, titanium oxide TiO 2 : 1.60 g, ammonium dihydrogen phosphate NH 4 h 2 (PO 4 ): 3.961 grams, after grinding and mixing evenly, select the first calcining in the air atmosphere, the temperature is 450 ° C, the pre-sintering time is 6 hours, and cool to room temperature. Take out the sample, grind it thoroughly again and mix it evenly, calcinate for the second time, and sinter at 1500°C for 2 hours in an air atmosphere, then cool down to 800°C at a rate of 20°C per hour, and then cool automatically to room temperature, and you can get Bulk barium titanium phosphate scintillation material. The main structural properties, X-ray excitation spectrum, excitation spectrum and luminescence spectrum are similar to those of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com