Indirect-competition-law enzyme linked immunosorbent assay kit for detecting phenylethanolamine A
An enzyme-linked immunosorbent reagent and phenylethanolamine technology, which is applied in measuring devices, instruments, scientific instruments, etc., to achieve the effects of low requirements for instruments and equipment, high accuracy and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Synthesis of Immunogen and Preparation of Immune Serum
[0023] 1.1 Reagents and instruments
[0024] Phenylethanolamine A (gifted by Hangzhou Dean Technology Co., Ltd.), succinic anhydride (purchased from Beijing Yaobei Biotechnology Co., Ltd.), pyridine (Pyridine, AR.WM=79.10, content > 99.5%, developed by Tianjin Kemiou Chemical Reagent center), N, N-dimethylformamide (Dimethylformamide, DMF, produced by ACROSORGANICS, New Jersey, USA, purchased from Bailingwei Chemical Reagent Company), EDC (purchased from Bailingwei Chemical Reagent Company), bovine serum albumin (BSA), Ovalbumin (OVA), etc. (purchased from Beijing Yaobei Biotechnology Co., Ltd.), and other reagents were of analytical grade.
[0025] Double-beam ultraviolet-visible spectrophotometer (TU-1909, Beijing Puxi General Instrument Co., Ltd.), chromatography device (3057 portable recorder, Chongqing Chuanyi No. 4 Factory; SBS series numerical control drop counter, constant flow pump, automatic p...
Embodiment 2
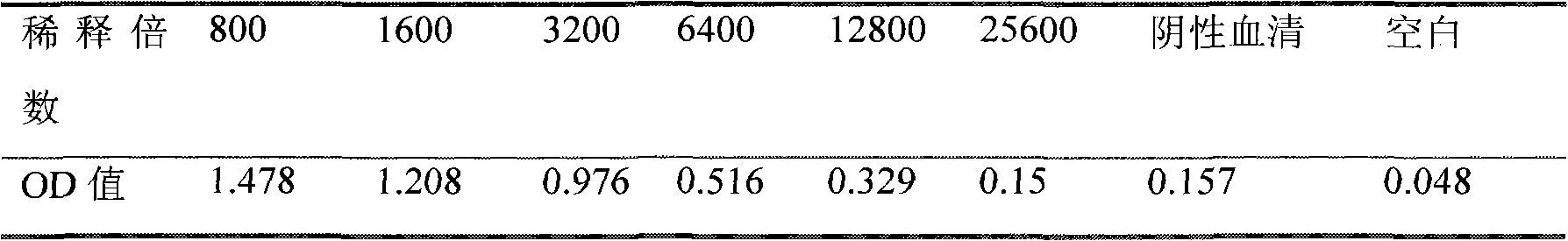
[0033] Example 2 Establishment of immunoassay method
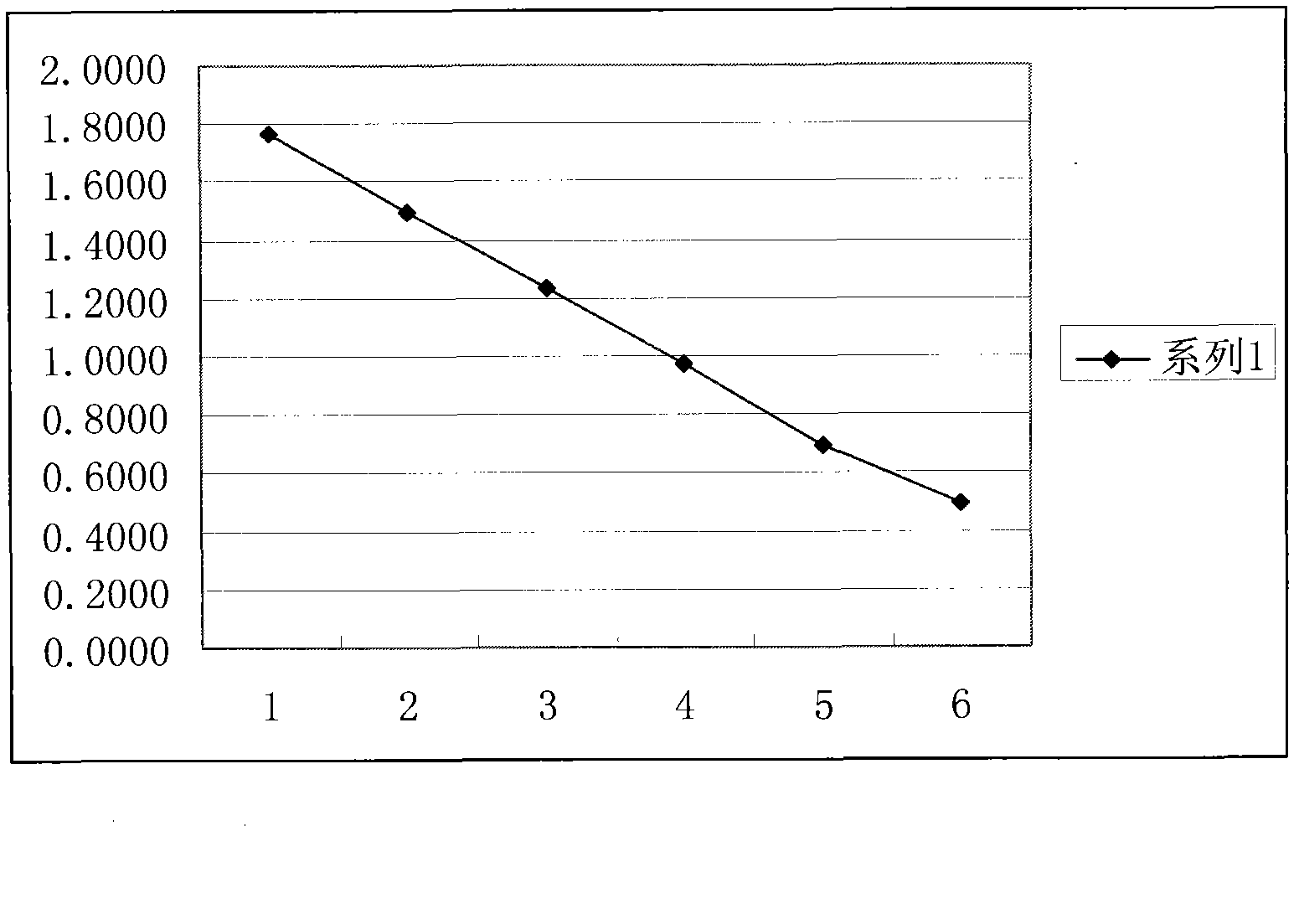
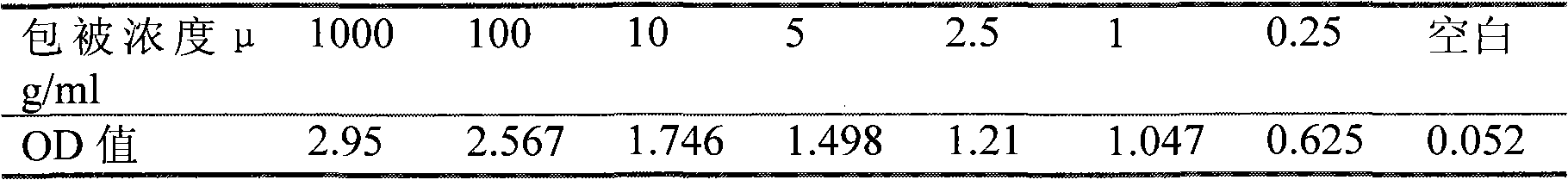
[0034] 2.1 ELISA method to determine the optimal coating concentration
[0035] Coat the enzyme plate with 100 μl per well of ovalbumin (OVA)-phenylethanolamine A conjugate at a series concentration of 1000 μg / ml, 100 μg / ml, 10 μg / ml, 5 μg / ml, 1 μg / ml, and 0.25 μg / ml , coated at 4°C for 24h, washed 5 times, patted dry, blocked with 200μl blocking solution per well for 12h at 4°C, washed 3 times, and patted dry. Add 100 μl of 1:500 diluted antiserum, react at room temperature for 2 hours, wash three times, immediately add 100 μl enzyme-labeled goat anti-rabbit antibody, react at room temperature for 30 minutes, wash three times, add 100 μl substrate solution, react at room temperature in the dark for 15 minutes, stop with 50 μl stop solution Reaction, microplate reader detects A value (450nm). At the same time, set blank control wells (without adding antiserum, only adding its diluent) and parallel repeat wells, and take ...
Embodiment 3
[0049] Example 3 Establishment of an enzyme-linked immunosorbent assay kit for detecting phenylethanolamine A
[0050] An enzyme-linked immunosorbent assay kit for detecting phenylethanolamine A was set up to include the following components:
[0051] (1) a microtiter plate coated with phenylethanolamine A antigen;
[0052] (2) Anti-phenylethanolamine A polyclonal antibody with a protein concentration of 1 mg / L;
[0053] (3) goat anti-rabbit anti-antibody labeled with horseradish peroxidase;
[0054] (4) 6 bottles of phenylethanolamine A standard solution, the concentrations are 0 μg / L, 1 μg / L, 5 μg / L, 10 μg / L, 50 μg / L, 100 μg / L;
[0055] (5) Substrate chromogenic solution A liquid is carbamide peroxide, and substrate chromogenic solution B liquid is tetramethylbenzidine;
[0056] (6) The washing liquid is a phosphate buffer containing 0.05% Tween 20;
[0057] (7) The concentrated sample diluent is a phosphate buffered saline solution of 0.1% Tween-20;
[0058] (8) The st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com