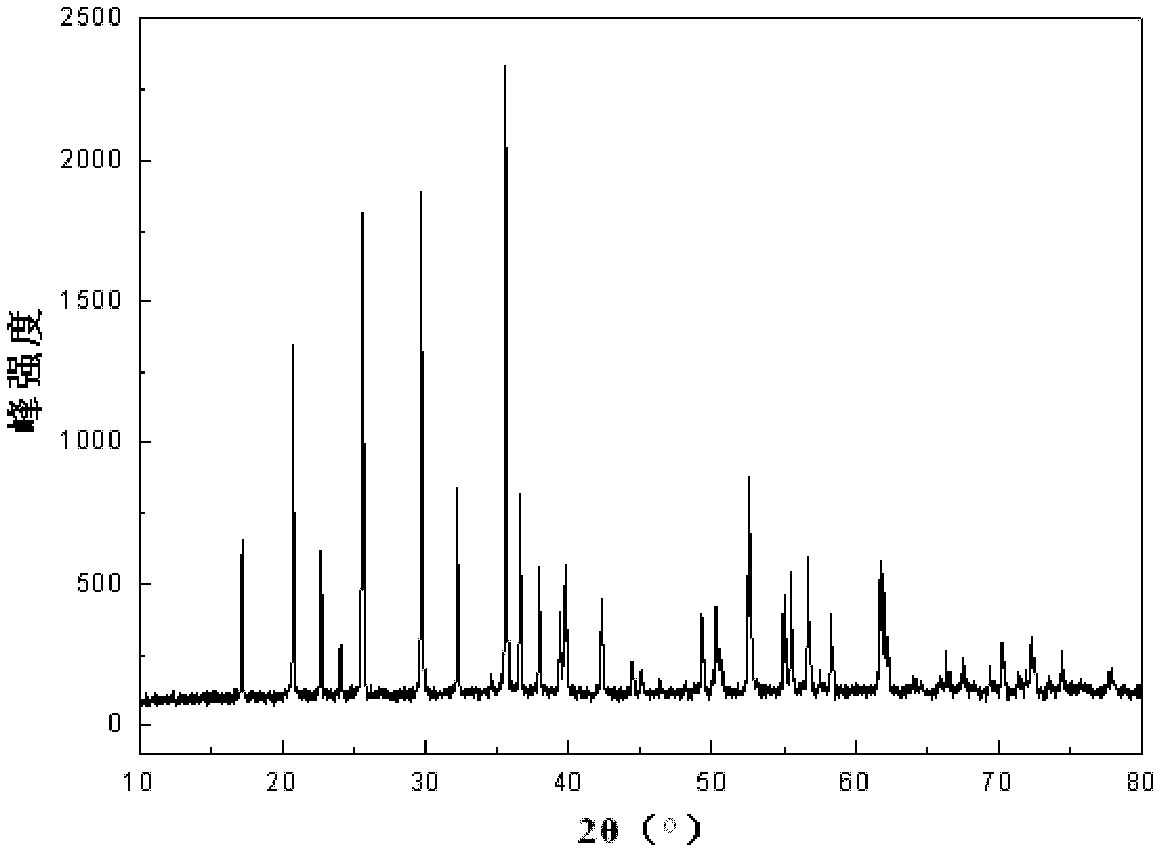
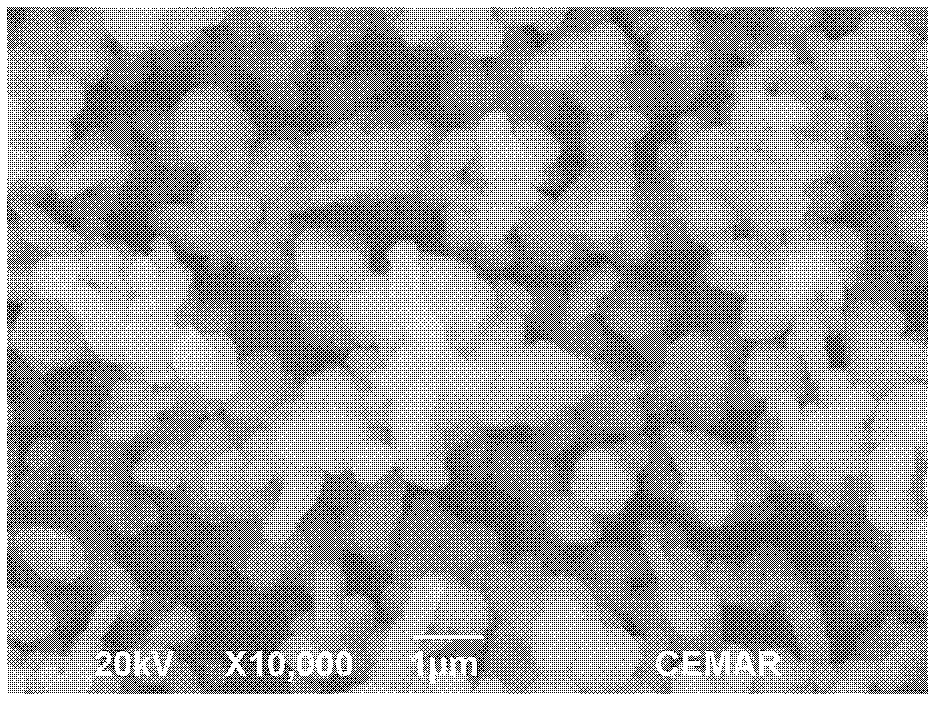
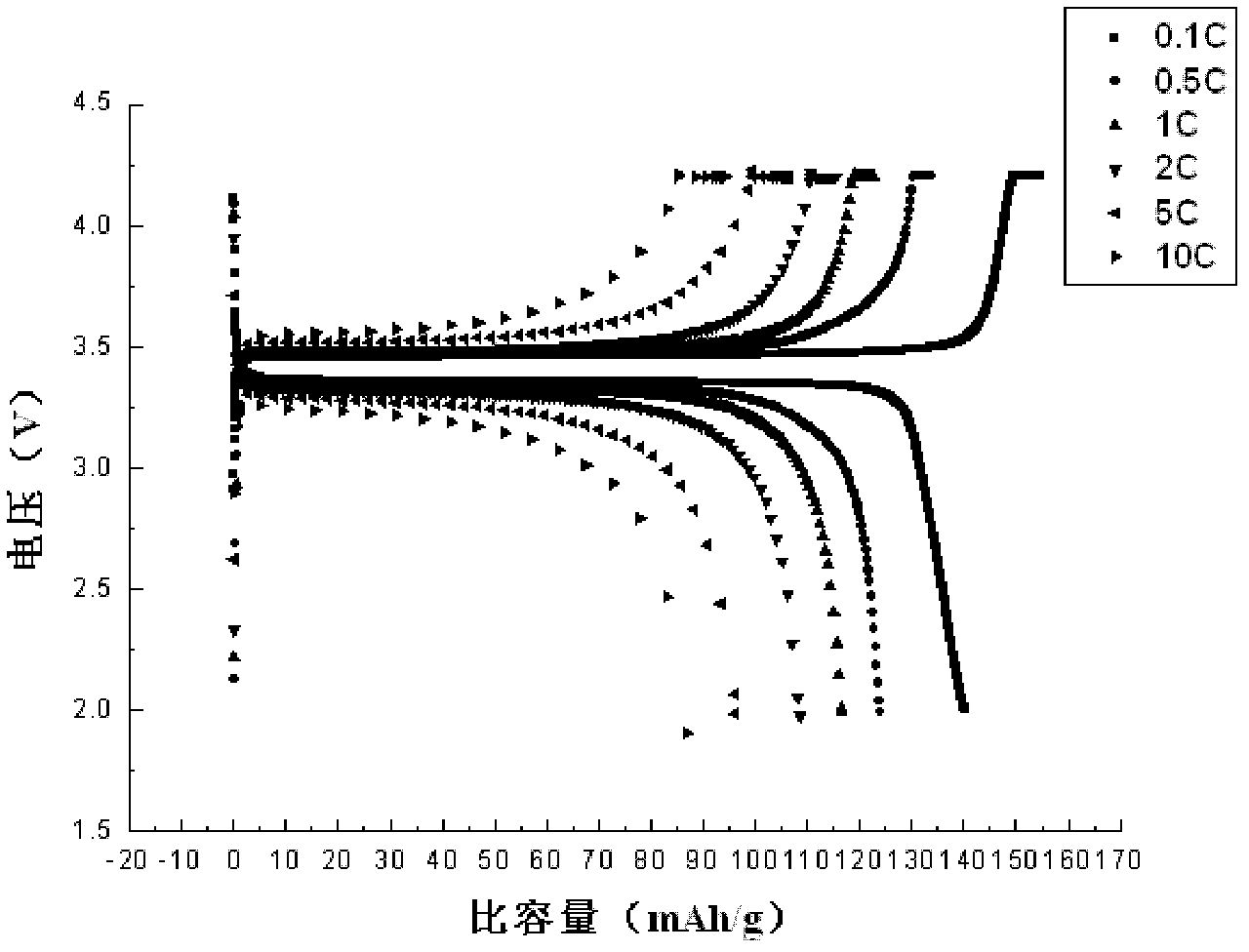
Method for preparing lithium iron phosphate cathode material of lithium ion battery by supercritical hydrothermal process
A lithium-ion battery, lithium iron phosphate technology, applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems of shortened reaction time, large particle size, insufficient charge and discharge performance, etc., to achieve shortened reaction time, small particles, The effect of excellent electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The first step, hydrothermal synthesis reaction
[0035] 5.56g of FeSO 4 ·7H 2 O was dissolved in 100 mL of deionized water; 2.306 g of 85% phosphoric acid was dissolved in 50 mL of deionized water; 2.569 g of LiOH·H 2 O was dissolved in 50 mL of deionized water.
[0036] Add the above phosphoric acid solution into the ferrous sulfate solution, stir and mix evenly; then add the lithium hydroxide solution into the mixed solution, finally add 0.3g of polyvinylpyrrolidone, and stir well. Transfer the mixed solution to a 500mL high-temperature and high-pressure reactor, seal the autoclave, use a vacuum pump to extract the air in the dead volume of the reactor, heat the high-temperature and high-pressure reactor to 400°C, adjust the internal pressure of the reactor to 25MPa through a water injection pump, and react for 1 minute. The proportioning control of reaction substance is: Li: Fe: P molar ratio is 3: 1: 1, before starting reaction, reactant concentration is counted...
Embodiment 2
[0049] The first step, hydrothermal synthesis reaction
[0050] 5.56g of FeSO 4 ·7H 2 O was dissolved in 100 mL of deionized water; 2.306 g of 85% phosphoric acid was dissolved in 50 mL of deionized water; 2.569 g of LiOH·H 2 O was dissolved in 50 mL of deionized water.
[0051] Add the above phosphoric acid solution into the ferrous sulfate solution, stir and mix evenly; then add the lithium hydroxide solution into the mixed solution, finally add 0.3g of cetyltrimethylammonium bromide, and stir well. Transfer the mixed solution to a 500mL high-temperature and high-pressure reactor, seal the autoclave, use a vacuum pump to extract the air in the dead volume of the reactor, heat the high-temperature and high-pressure reactor to 380°C, adjust the internal pressure of the reactor to 25MPa through a water injection pump, and react for 10 minutes. The proportioning control of reaction substance is: Li: Fe: P molar ratio is 3: 1: 1, before starting reaction, reactant concentratio...
Embodiment 3
[0058] The first step, hydrothermal synthesis reaction
[0059] 5.56g of FeSO 4 ·7H 2 O was dissolved in 100 mL of deionized water; 2.306 g of 85% phosphoric acid was dissolved in 50 mL of deionized water; 2.569 g of LiOH·H 2 O was dissolved in 50 mL of deionized water.
[0060] Add the above-mentioned phosphoric acid solution into the ferrous sulfate solution, stir and mix evenly; then add the lithium hydroxide solution into the mixed solution, finally add 0.2g of polyvinylpyrrolidone, and stir well. Transfer the mixed solution to a 500mL high-temperature and high-pressure reactor, seal the autoclave, use a vacuum pump to pump out the air in the dead volume of the reactor, heat the high-temperature and high-pressure reactor to 400°C, adjust the internal pressure of the reactor to 30MPa through a water injection pump, and react for 1 minute. The proportioning control of reaction substance is: Li: Fe: P molar ratio is 3: 1: 1, before starting reaction, reactant concentration...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com